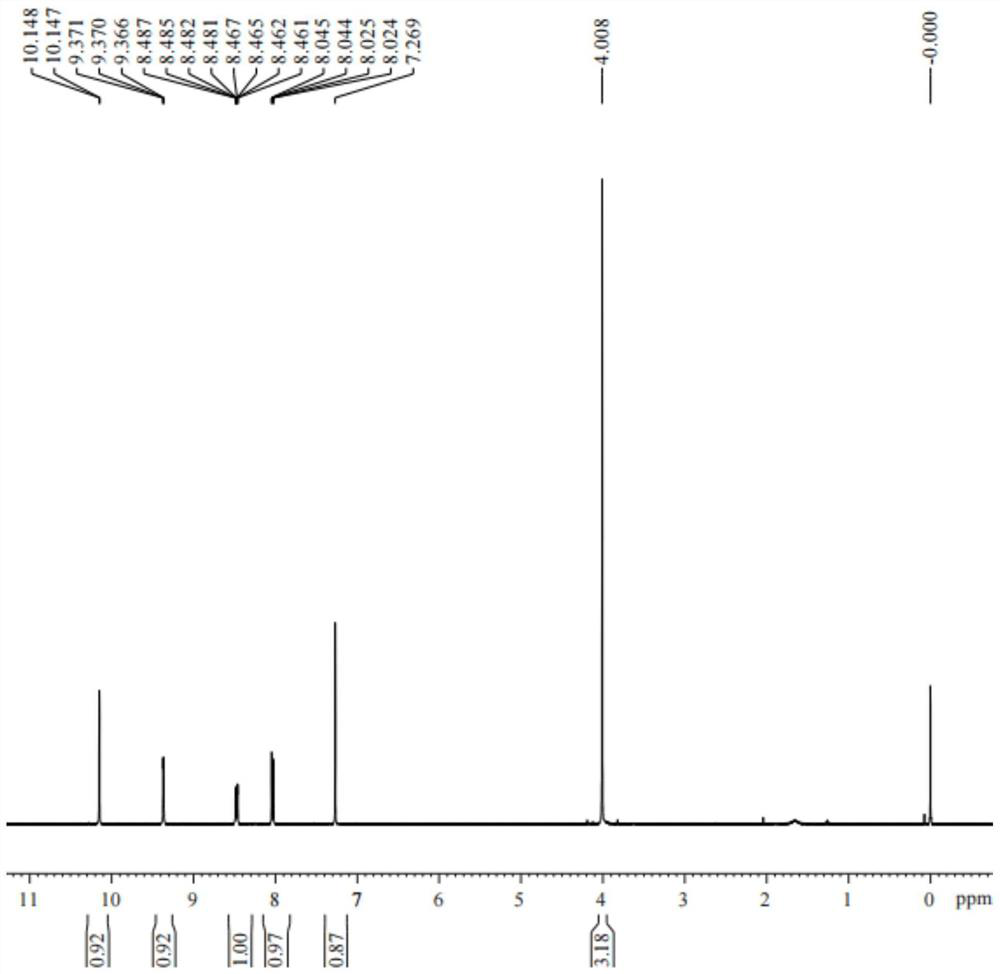
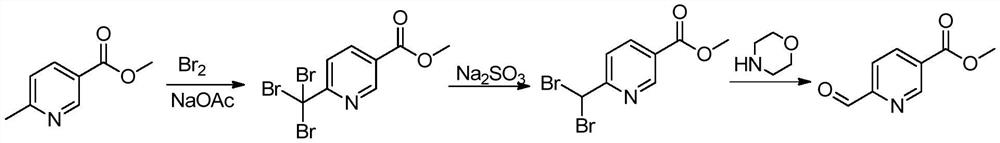
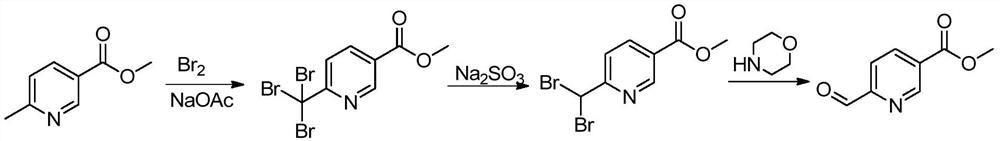
Synthesis method of 6-formyl methyl nicotinate
A technology of methyl formyl nicotinate and methyl methyl nicotinate, which is applied in the field of synthesis of methyl 6-formyl nicotinate, can solve the problems of expensive raw materials, high cost, pollution, etc., and achieve low raw material cost, Easy to operate and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of synthetic method of 6-formyl nicotinic acid methyl ester, its concrete steps are as follows:
[0026] (1): Add glacial acetic acid (1000L), methyl 6-methylnicotinate (100kg) and anhydrous sodium acetate (218kg) successively in the dry reaction kettle, heat up to 50°C, add bromine (339kg) dropwise, The reaction was stirred for 18 hours after dropping; after the reaction, the reaction liquid was lowered to room temperature, filtered, and the obtained solid was washed with a large amount of water and dried to obtain methyl 6-tribromomethylnicotinate (200kg).
[0027] (2): Methanol (300L), ethyl acetate (750L) and methyl 6-tribromomethylnicotinate (150kg) were sequentially added to the dry reactor, stirred at room temperature, and sodium sulfite (150kg) was added dropwise in water (750L ) solution, keep stirring at 25°C for 1 hour after dripping; filter the reaction solution, let the filtrate stand for stratification, wash the organic phase with salt water, dry wi...
Embodiment 2
[0030] A kind of synthetic method of 6-formyl nicotinic acid methyl ester, its concrete steps are as follows:
[0031] (1): Add glacial acetic acid (90L), methyl 6-methylnicotinate (10kg) and anhydrous sodium acetate (18kg) in turn to the dry reaction kettle, heat up to 50°C, add bromine (31.7kg) dropwise , stirring and reacting for 18 hours after dripping; the reaction solution was lowered to room temperature, filtered, and the obtained solid was washed with a large amount of water and dried to obtain methyl 6-tribromomethyl nicotinate (18.5kg).
[0032] (2): Add methanol (27L), ethyl acetate (72L) and methyl 6-tribromomethylnicotinate (15kg) to the dry reaction kettle in turn, stir at room temperature, add sodium sulfite (13kg) dropwise in water (65L ) solution, keep stirring at 25°C for 1 hour after dripping; filter the reaction solution, let the filtrate stand for stratification, wash the organic phase with salt water, dry with anhydrous sodium sulfate, and concentrate the...
Embodiment 3
[0035] A kind of synthetic method of 6-formyl nicotinic acid methyl ester, its concrete steps are as follows:
[0036] (1): Add glacial acetic acid (120L), methyl 6-methylnicotinate (10kg) and anhydrous sodium acetate (26kg) in turn to the dry reaction kettle, heat up to 50°C, add bromine (35.8kg) dropwise , and stirred for 18 hours after dropping; the reaction solution was lowered to room temperature, filtered, and the obtained solid was washed with a large amount of water and dried to obtain methyl 6-tribromomethylnicotinate (19.5kg).
[0037] (2): Methanol (33L), ethyl acetate (78L) and methyl 6-tribromomethylnicotinate (15kg) were sequentially added to the dry reactor, stirred at room temperature, and sodium sulfite (17kg) was added dropwise in water (85L ) solution, keep stirring at 25°C for 1 hour after dripping; filter the reaction solution, let the filtrate stand for stratification, wash the organic phase with salt water, dry with anhydrous sodium sulfate, and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com