Compositions, devices, and methods for treatment of overdose and reward-based disorders
A formulation, volumetric technique, applied in the field of administration of an intranasal formulation of the opioid antagonist naltrexone, in the treatment of conditions such as opioid overdose and its symptoms and disorders such as alcohol use disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
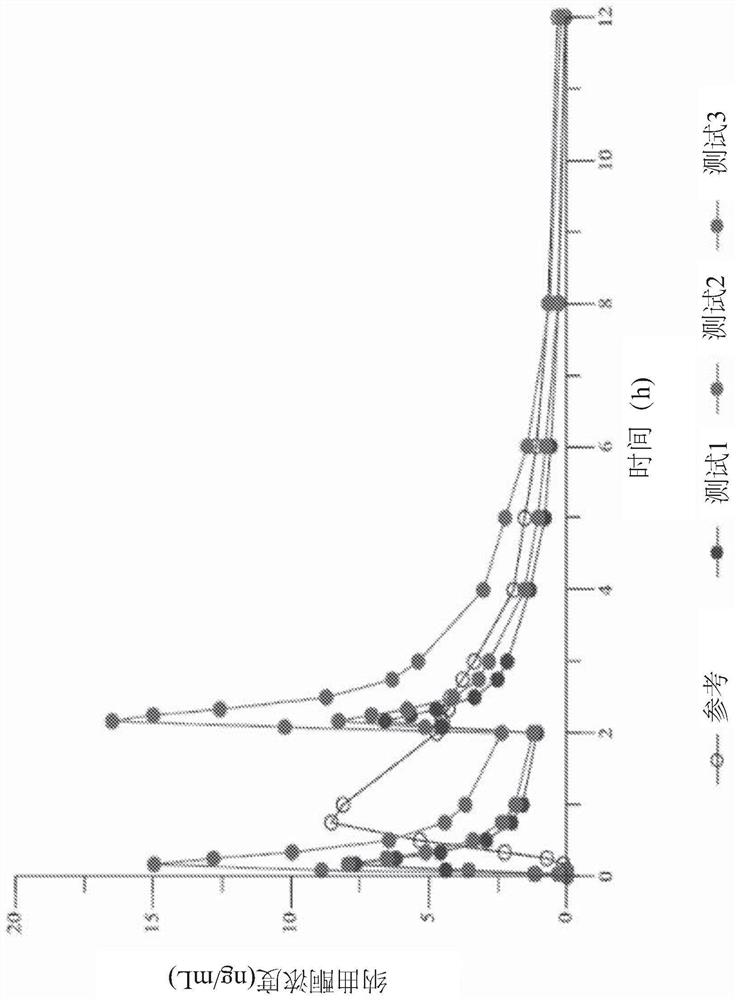
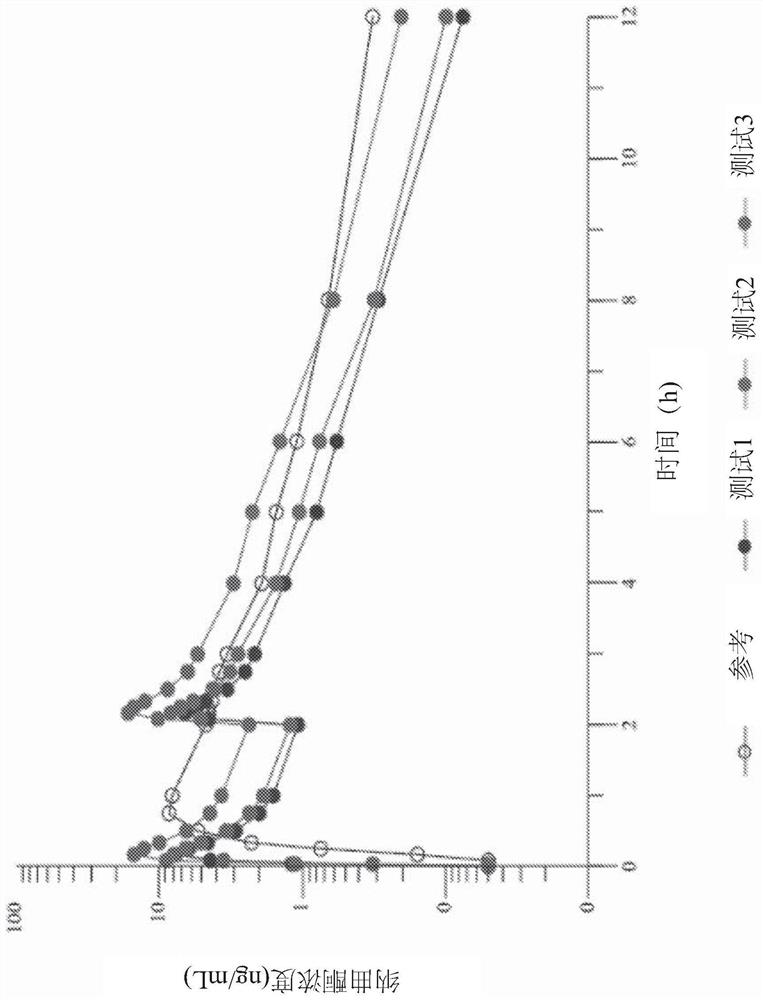
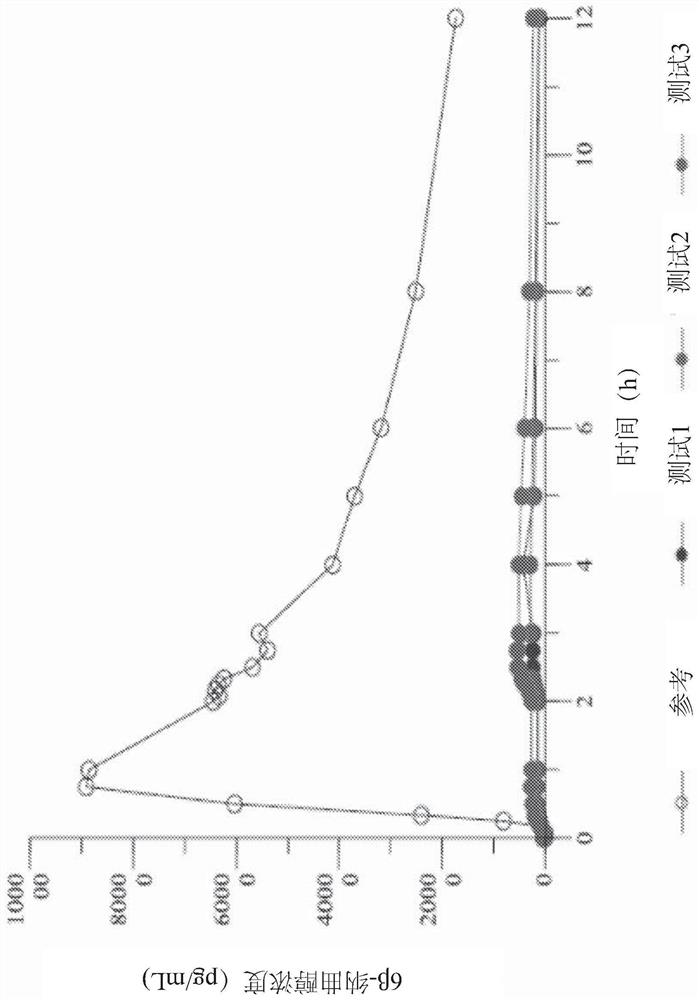
Examples
Embodiment approach 3
[0026] Embodiment 3: according to the preparation described in embodiment 1 or embodiment 2, it further comprises:
[0027] Isotonic agent;
[0028] preservative;
[0029] stabilizer;
[0030] absorption enhancers; and
[0031]An amount of water sufficient to achieve a final volume of about 50 to about 250 μL, preferably about 50 to about 150 μL.
Embodiment approach 4
[0032] Embodiment 4: The formulation according to embodiment 3, comprising:
[0033] about 1 mg to about 3 mg of naltrexone or a pharmaceutically acceptable salt thereof;
[0034] about 0.1 mg to about 1.2 mg of an isotonic agent;
[0035] from about 0.001 mg to about 0.1 mg of a preservative;
[0036] from about 0.1 mg to about 0.5 mg of a stabilizer;
[0037] about 0.05 mg to about 2.5 mg of an absorption enhancer; and
[0038] An amount of water sufficient to achieve a final volume of about 50 to about 250 μL, preferably about 50 to about 150 μL.
Embodiment approach 5
[0039] Embodiment 5: The formulation according to embodiment 3, comprising:
[0040] about 1% to about 3% naltrexone or a pharmaceutically acceptable salt thereof;
[0041] about 0.1% to about 1.2% isotonic agent;
[0042] from about 0.001% to about 0.1% preservatives;
[0043] from about 0.1% to about 0.5% stabilizer;
[0044] From about 0.05% to about 2.5% absorption enhancers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bmi | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com