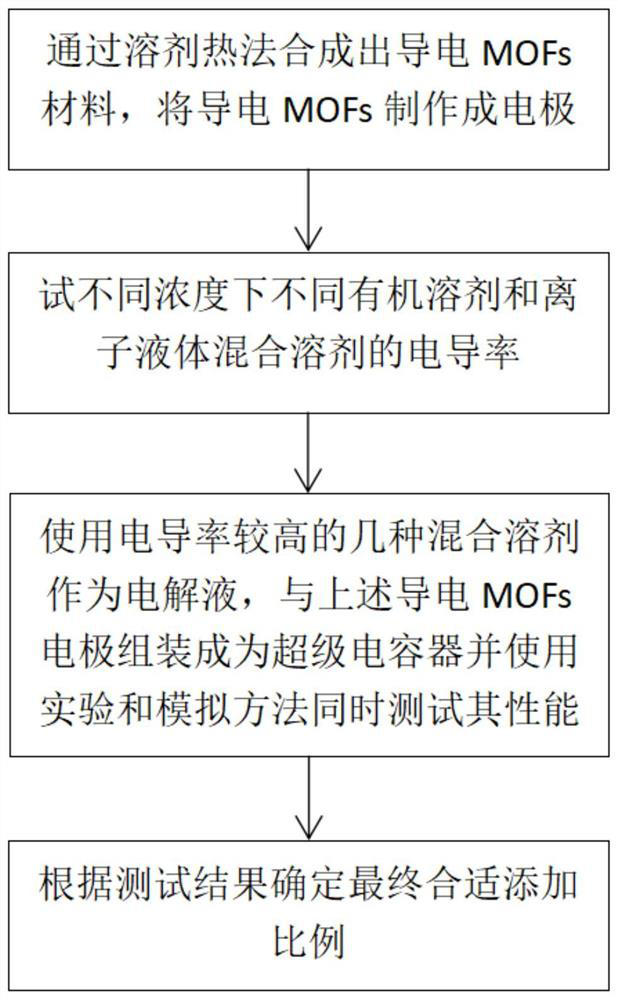
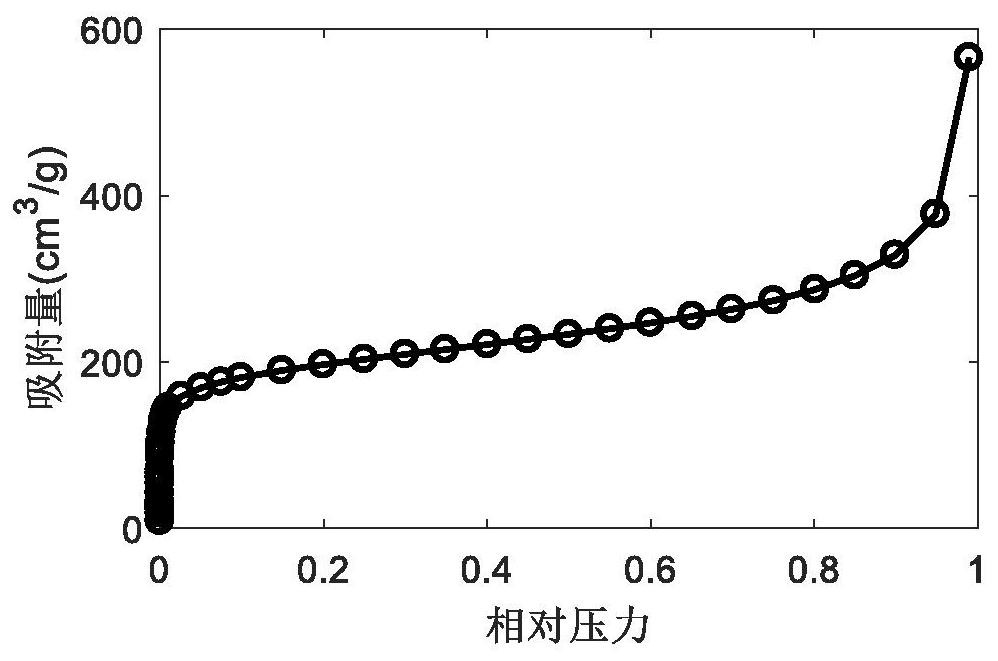
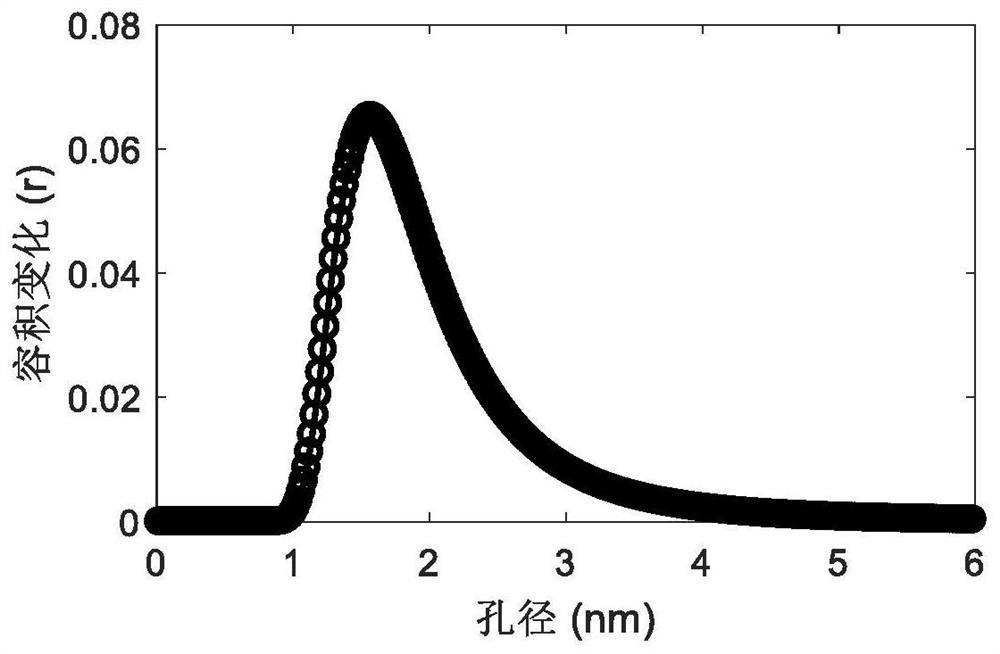
A kind of mofs ionic liquid supercapacitor and preparation method thereof
A supercapacitor, ionic liquid technology, applied in the manufacture of hybrid/electric double layer capacitors, hybrid capacitor electrodes, etc., can solve the problems of reducing energy density, inability to improve, limiting supercapacitor power density, etc., to achieve energy density and power density improvement , easy to study, increase the effect of capacitance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Using the same Ni as in Comparative Example 1 3 (HITP) 2 Powders are made into electrodes.
[0049] (2) Use a conductivity meter to test the conductivity of the mixed solvent when adding different concentrations of organic solvents, such as Image 6 As shown, the ionic liquid used in this example is 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF 6 ]), and the organic solvent used was acetonitrile (AN).
[0050] (3) Use [BMIM][PF 6 A mixed solvent with a molar ratio of ] / AN of 0.107 was used as the electrolyte to assemble a supercapacitor with a conductive MOF electrode. The energy density, power density, capacitance, equivalent series resistance / internal resistance and other parameters were measured by electrochemical means. The measurement results are shown in Table 1 and Figure 8 to Figure 10 As shown, after adding an organic solvent with a ratio of 0.107, the energy density and power density of the supercapacitor are 276% and 1263% of those of p...
Embodiment 2
[0052] (1) Using the same Ni as in Comparative Example 1 3 (HITP) 2 Powders are made into electrodes.
[0053] (2) Use a conductivity meter to test the conductivity of the mixed solvent when adding different concentrations of organic solvents, such as Image 6 As shown, the ionic liquid used in this example is 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF 6 ]), and the organic solvent used was acetonitrile (AN).
[0054] (3) Use [BMIM][PF 6 A mixed solvent with a molar ratio of ] / AN of 0.2 was used as the electrolyte to assemble a supercapacitor with a conductive MOF electrode. The energy density, power density, capacitance, equivalent series resistance / internal resistance and other parameters were measured by electrochemical means. The measurement results are shown in Table 1 and Figure 8 to Figure 10 As shown, after adding an organic solvent with a ratio of 0.2, the energy density and power density of the supercapacitor are 344.7% and 1096% of those of pur...
Embodiment 3
[0058] (1) Using the same Ni as in Comparative Example 2 3 (HITP) 2 Powders are made into electrodes.
[0059] (2) Use a conductivity meter to test the conductivity of the mixed solvent when adding different concentrations of organic solvents, such as Figure 7 As shown, the ionic liquid used in this example is 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF 4 ]), and the organic solvent used was acetonitrile (AN).
[0060] (3) Use [EMIM][BF 4 A mixed solvent with a molar ratio of ] / AN of 0.107 was used as the electrolyte to assemble a supercapacitor with a conductive MOF electrode. Using electrochemical means to measure its energy density, power density, capacitance, equivalent series resistance / internal resistance and other parameters, the measurement results are as follows Figure 8 to Figure 10 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap