Kit for detecting gene mutation of aminoglycoside drugs
An aminoglycoside and kit technology, used in genomics, microbial determination/inspection, bioinformatics, etc., can solve the problems of limited number of samples tested, high sequencing cost, difficulty in interpretation of results, etc., to achieve low cost, sustainable The effect of high sensitivity and specificity, easy to popularize and apply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kit for detecting gene mutations of aminoglycoside drugs according to this embodiment, the kit includes the probes shown in SEQ ID No.1~SEQ ID No.2.
[0047] The design of the probe is combined with the literature reports of clinical laboratories, aiming at the clinical diagnosis and treatment guidelines related to aminoglycoside drug genes, combined with databases such as ClinVar, CPIC and pharmaGKB to screen these sites, and confirmed 2 high-incidence sites in the Chinese population , and synthesize the corresponding probes for these two sites and make a chip, with one probe for each site. The screening principles are as follows:
[0048] (1) High-incidence sites in the Chinese population included in clinical drug guidelines;
[0049] (2) The ClinVar database is rated as Drug response, and the evidence strength is 3 stars or above;
[0050] (3) The CPIC and pharmaGKB databases publish Guidelines and the CPIC grade is A.
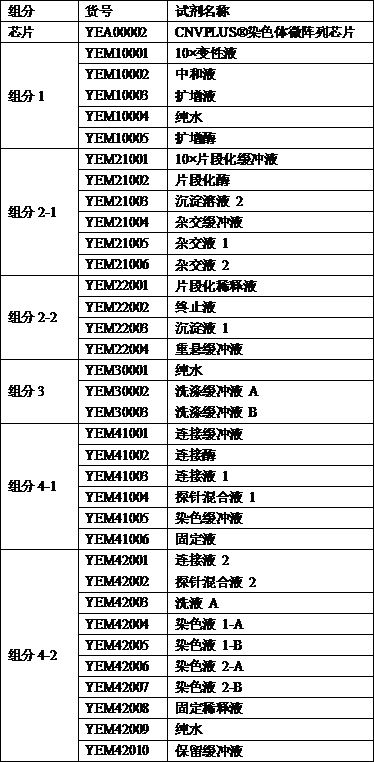
[0051] The components of the kit also inc...
Embodiment 2
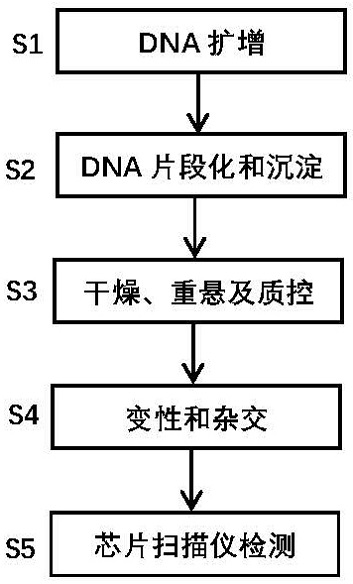
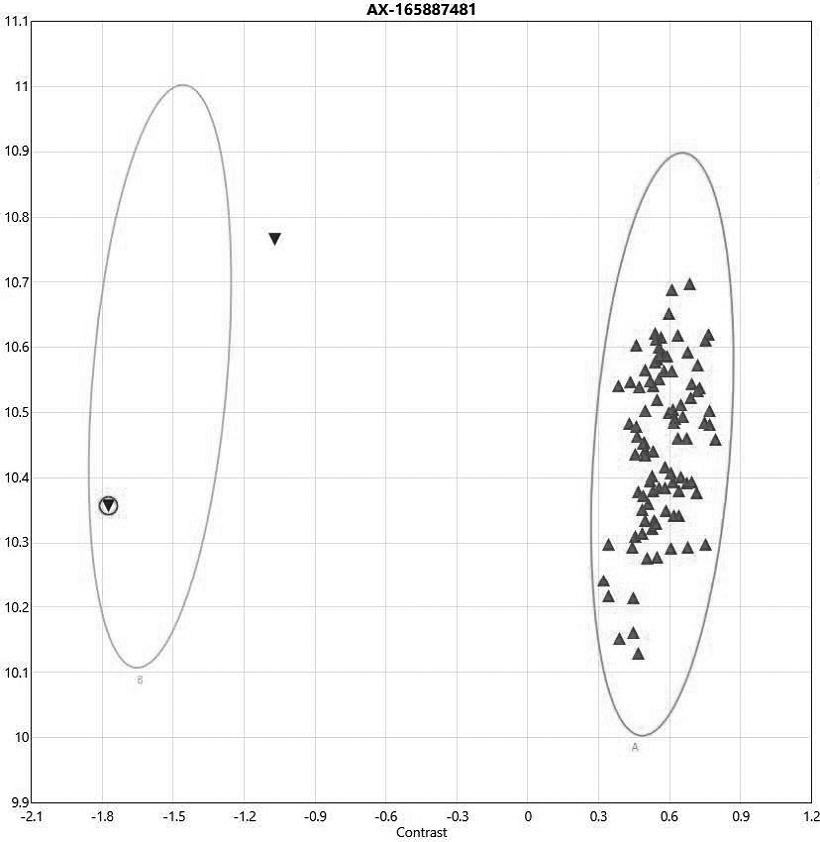
[0056] In the present embodiment, clinical samples are actually detected by the present invention, which specifically includes the following steps:
[0057] (1) Sample processing and quality control: 2 clinically obtained samples were used as positive verification samples for the invention. These verification samples are first subjected to DNA extraction, and quality control is carried out through gel electrophoresis, Nanodrop, etc. to ensure that each DNA sample has no degradation, no impurity contamination, and high purity. The optical density (Optical Density, OD) 260 / 280nm ratio is between 1.8~2.0, and the OD 260 / 230nm ratio is between 1.5~2.0. Samples that do not meet any of the conditions need to be purified and other treatments. The gel loading dye is Invitrogen™ TrackIt Cyan / Orange Loading Buffer (Invitrogen P / N 10482-028); 25bp Invitrogen Ladder (Invitrogen P / N 10488-022); the gel electrophoresis system is Invitrogen E-Gel™ 48 agarose gels 4%, G8008-04.
[0058] (2)...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap