Dose regimen for administration of LAG-3/PD-L1 bispecific antibodies
A technology of PD-L1 and LAG-3, applied in the direction of antibodies, antibody medical components, specific peptides, etc., can solve the problems that LAG-3/PD-L1 antibodies have not yet been obtained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0218] Example 1: First in human (FIH) dose justification and dose escalation strategy for FS118 slightly
[0219] FS118 is a bispecific antibody molecule that simultaneously targets two immune checkpoint proteins, LAG-3 and PD-L1. FS118 has been shown to differ from monospecific immune checkpoint inhibitors, such as anti-PD-L1 antibodies, in a number of important ways. These differences require detailed analysis to determine the appropriate dose for Phase I studies of FS118 in human patients. Specifically, FS118 was tested in in vitro and in vivo studies to determine the optimal starting dose and dose escalation strategy for a phase I human study designed to determine the efficacy of FS118 in patients with previously PD-containing Safety, tolerability, pharmacokinetics and activity in patients with advanced malignancies who have progressed on or after 1 / PD-L1 therapy (see Example 2 below).
[0220] 1.1 FS118 and mLAG-3 / PD-L1: Overview of nonclinical studies
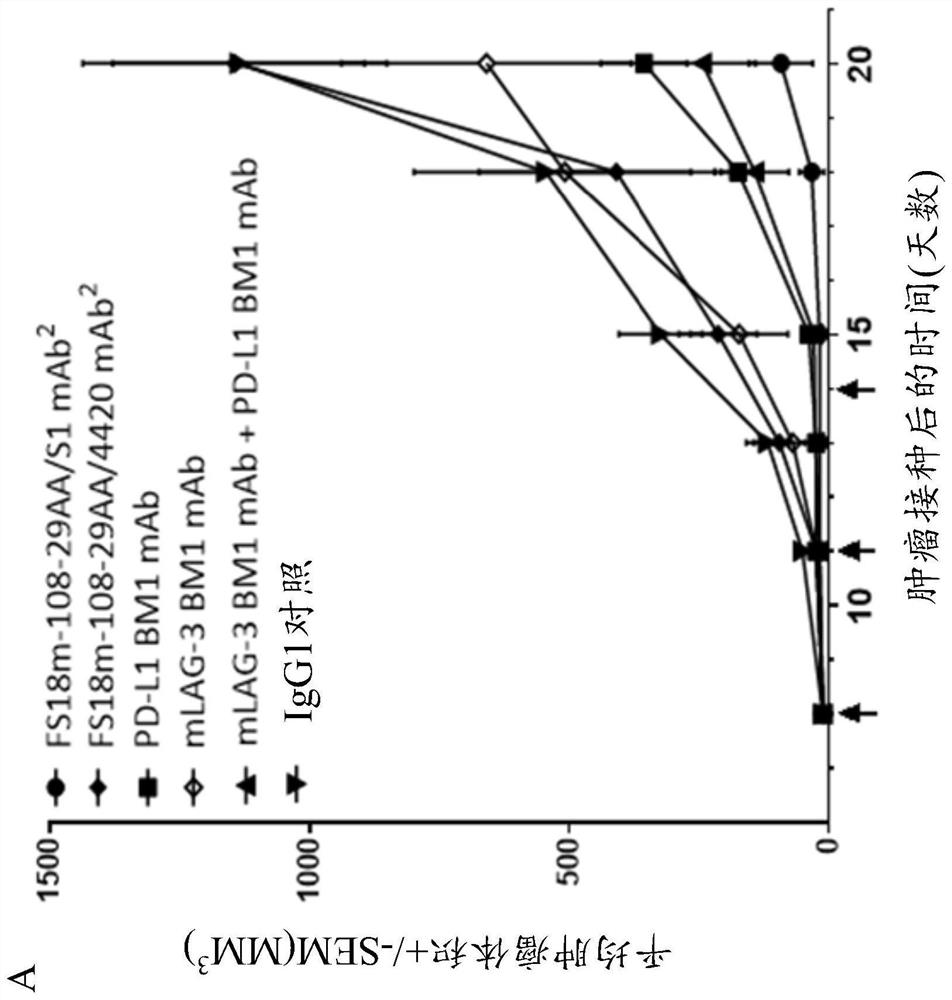
[0221] ...
Embodiment 2
[0330] Example 2: LAG-3 / PD-L1 bispecific antibody FS118 on or during prior PD-1 / PD-L1 containing therapy Phase I, open-label, safety, tolerability, pharmacokinetics, and activity in patients with advanced malignancies after progression Dose Escalation and Cohort Expansion First-in-Human Study
[0331] 2.1 Study Design and Parameters
[0332]Studies were conducted in adult patients diagnosed with advanced tumors to characterize the safety, tolerability, pharmacokinetics (PK) and activity of FS118. This phase I, multicenter, open-label, multiple-dose, first-in-human study was initiated with an accelerated titration design (over which a single patient cohort was evaluated) followed by a 3+3 ascending dose escalation design. The study was designed to systematically assess the safety and tolerability, maximum tolerated dose (MTD) and / or phase II recommended dose (RP2D) of FS118 in patients with confirmed advanced tumors. RP2D is defined as the maximum biologically effectiv...
Embodiment 3
[0490] Example 3: Selection of those more likely to respond to FS118 based on resistance to prior anti-PD-1 or anti-PD-L1 therapy patient
[0491] 3.1 Background
[0492] All patients included in the ongoing FS118 trial progressed on or after PD-1 / PD-L1-containing therapy.
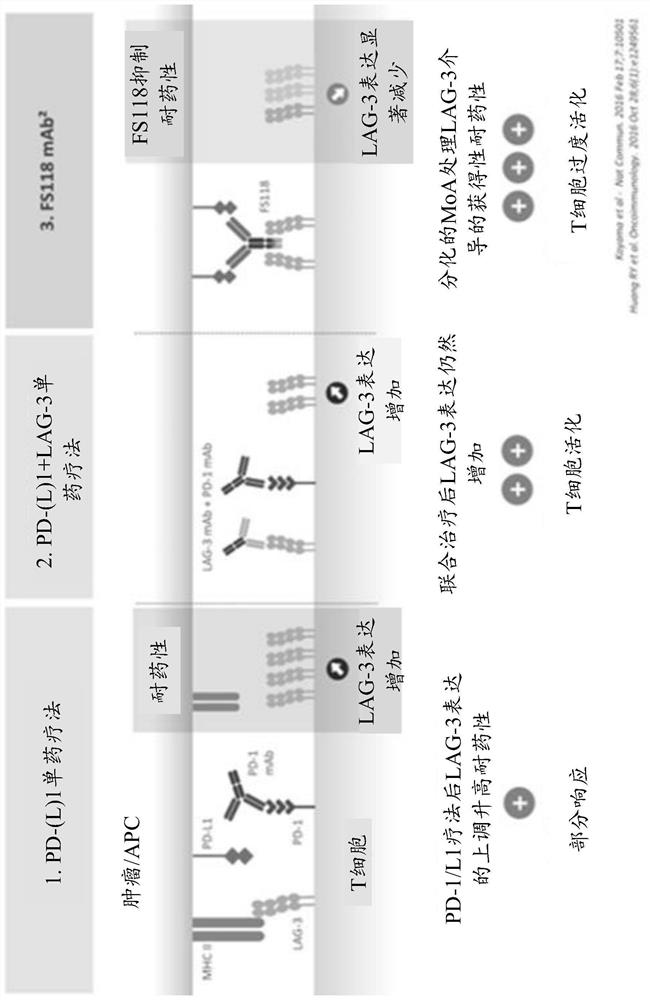
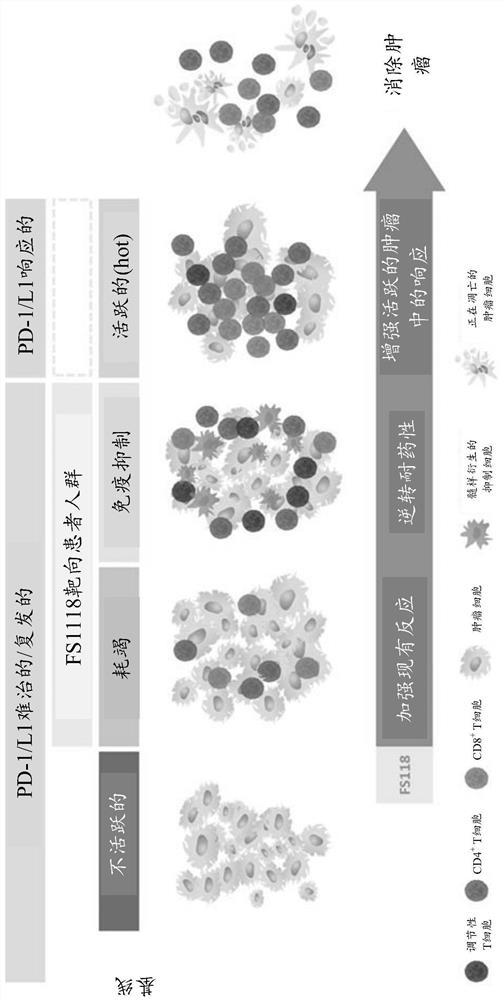
[0493] Initial results (August 2019) demonstrated that FS118 was able to stabilize disease in some patients with a disease control rate (DCR) of 34.4% (see Example 2.3.1), rising to 47.2% as of April 2020 (see Implementation Example 2.4.1). The inventors hypothesized that FS118 could provide benefit to these patients due to the combination of a LAG-3 inhibitor with a PD-L1 inhibitor (dual checkpoint inhibitor) or by a PD-L1 and LAG-3 bispecific Additional benefits provided by targeting novel biology (WO2017220569A1). Patients are not expected to realize clinical benefit on retreatment with anti-PD-1 or anti-PD-L1-containing regimens alone (Fujita et al., Anticancer Res. (2019); Fujita et al., Thor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com