Kit for detecting SCN1A gene copy number variation
A gene copy number, SCN1A.1.R technology, applied in the field of biomedical detection, can solve the problem of not finding the report of SCN1A gene copy number variation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
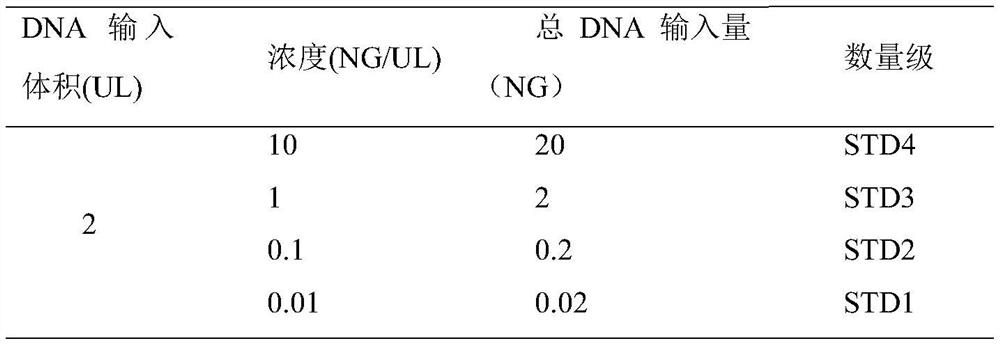
[0048] 1. Primer test
[0049] 1) Linear DNA preparation (Table 1): serially dilute the standard HUMAN GENETIC DNA (253NG / UL), take 1UL and add 24.3UL DDH 2 O, vortex, micro separation, the concentration is 10NG / UL.
[0050] Then 10-fold serial dilution, take 10UL and add to 90UL DDH 2 In O, it is necessary to fully vortex and mix each time before proceeding to the next gradient dilution.
[0051] Table 1
[0052]
[0053]
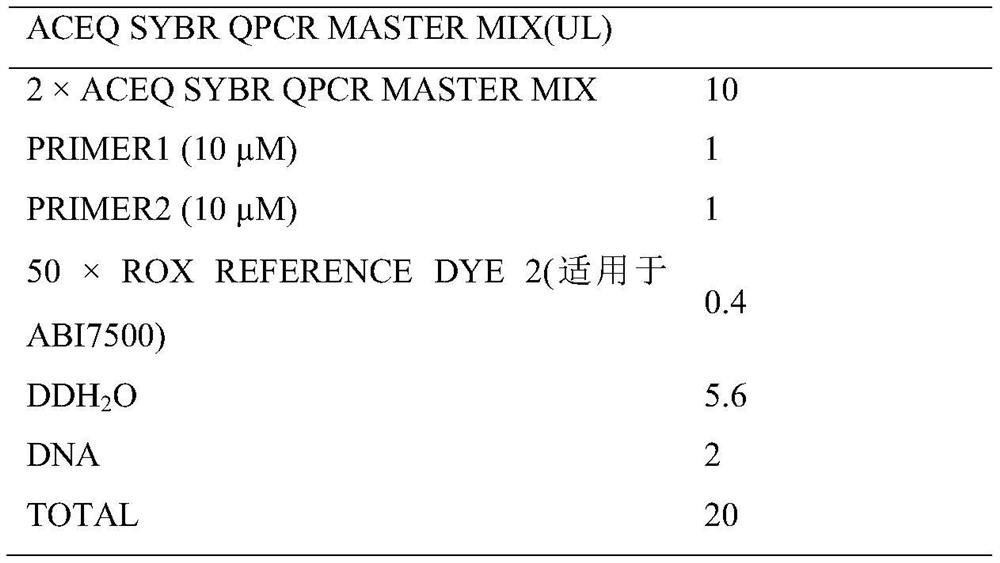
[0054] 2) Experimental system (Table 2)
[0055] Table 2
[0056]
[0057] 3) Amplification program (Table 3)
[0058] table 3
[0059]
[0060] 4) Analysis of results
[0061] ABI7500 settings:
[0062] EXPERIMENT PROP chooses QUANTUTATION–RELATIVE STANDARD CURVE AND SYBRGREEN REAGENTS.
[0063] The result is as follows:
[0064] Primer SCN1A-1: the amplification efficiency of the primer is 104.87%, and the peak of the melting curve is single;
[0065] Primer SCN1A-2: The amplification efficiency of the primer is 90.89%, and the pea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com