Compositions and methods for treating central nervous system disorders
A composition and barrier technology, which is applied in the directions of non-active components of polymer compounds, drug delivery, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0232] Example 1 : Composition for intranasal delivery
[0233] The following compositions were prepared using standard mixing equipment and procedures.
[0234]
[0235] *Based on suramin hexasodium salt having a molecular weight of 1429.15 g / mol
[0236] Dissolve suramin sodium salt in water by mixing gently. Add cyclodextrin with mixing until dissolved. The resulting solution was allowed to stand for 2 hours before use.
[0237] The composition can be packaged in a spray bottle for intranasal administration.
[0238] Alternatively, compositions were prepared substituting an equal weight of caprylic caprate macrogol-8 glyceride or 2-(2-ethoxyethoxy)ethanol for methyl beta-cyclodextrin.
[0239] The compositions are useful in the treatment of autism spectrum disorders.
Embodiment 2
[0240] Example 2 : Composition for intranasal delivery
[0241] The following compositions were prepared using standard mixing equipment and procedures.
[0242]
[0243] *Based on suramin hexasodium salt having a molecular weight of 1429.15 g / mol
[0244] Dissolve suramin sodium salt in water by mixing gently. Sodium chloride and hydroxypropyl methylcellulose were added with mixing. Add cyclodextrin with mixing until dissolved. The resulting solution was allowed to stand for 2 hours before use.
[0245] The composition can be packaged in a spray bottle for intranasal administration.
[0246] Alternatively, compositions were prepared substituting an equal weight of caprylic caprate macrogol-8 glyceride or 2-(2-ethoxyethoxy)ethanol for methyl beta-cyclodextrin.
[0247] The compositions are useful in the treatment of autism spectrum disorders.
Embodiment 3
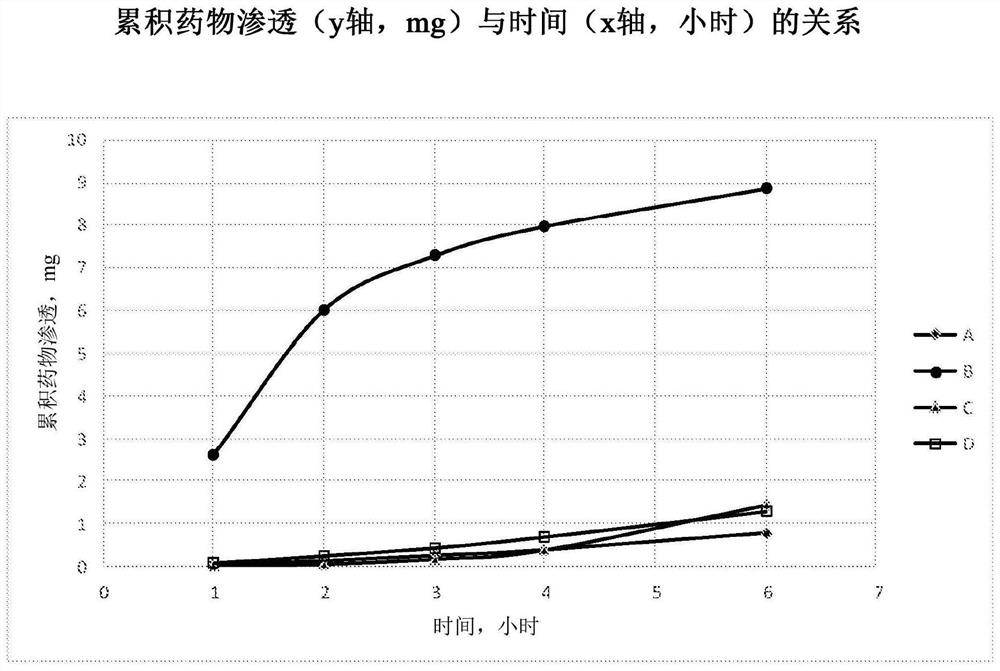
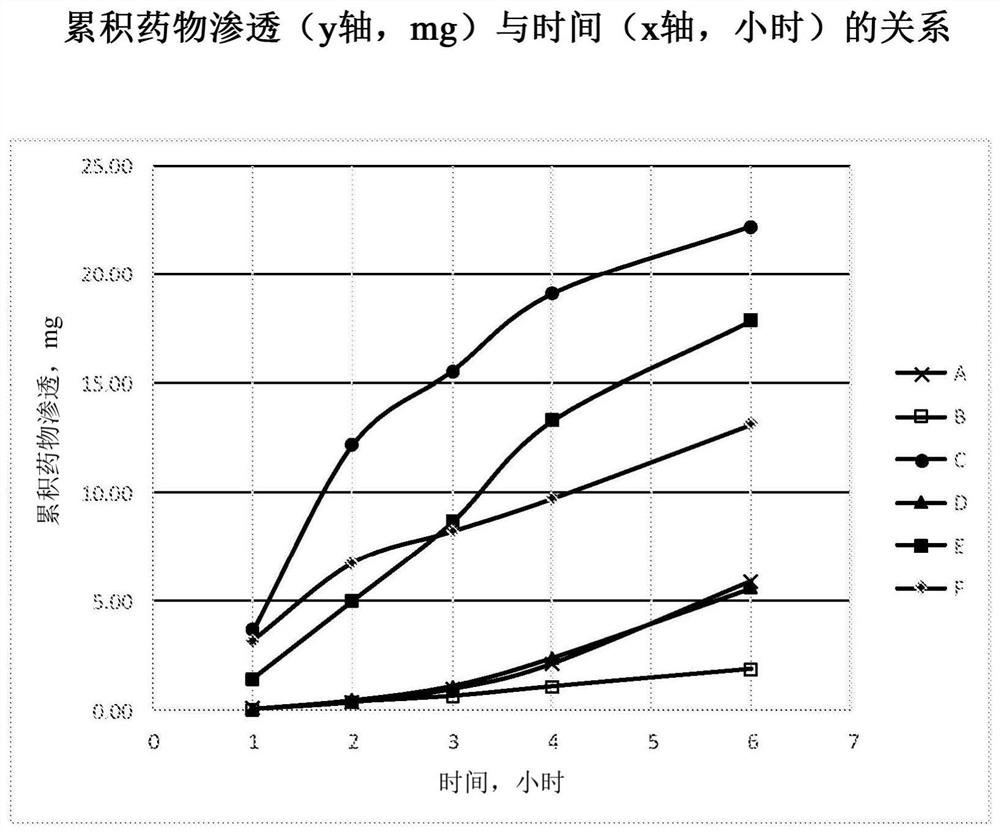
[0248] Example 3 : Tissue penetration of suramin
[0249] Four formulations A-D were prepared using the methods of Examples 1 and 2 and were found to be stable for at least 4 weeks over a three month period at 25°C and 60% relative humidity.
[0250] Formulation A - 100 mg / mL suramin hexasodium salt in water (no excipients)
[0251] Formulation B - 100 mg / mL suramin hexasodium salt in water with 40% methyl beta-cyclodextrin (Methy betadex)
[0252] Formulation C - 100 mg / mL Suramin Hexasodium Salt in Water with 40% HP(Hydroxypropyl)-Cyclodextrin
[0253] Formulation D - 160 mg / mL suramin hexasodium salt in water (no excipients)
[0254] These formulations also contained 0.1% hydroxypropylmethylcellulose (ie, HPMC E5 from Dow Chemicals) as a solution thickener; and 0.75% sodium chloride as an osmolarity agent.
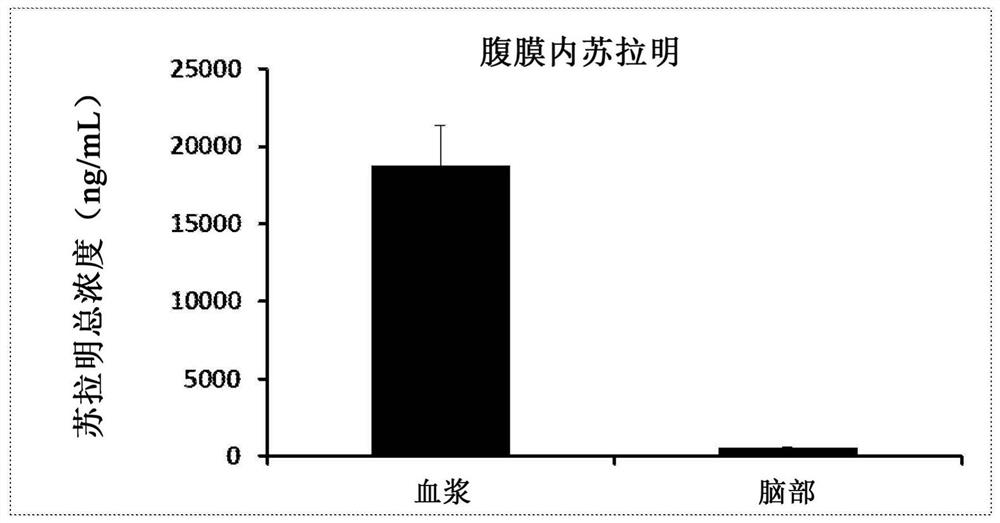
[0255] These four formulations were evaluated in an in vitro permeation study using cultured human airway tissue (EpiAirway AIR-100, purchased from MatTek Corpor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com