Use of ink pterin and metabolites thereof to treat radiation exposure
A technique for mopterin, biopterin, used in the field of treatment of subjects exposed to radiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1. Treatment of C57L / J mouse group
[0102] animal
[0103] Both male and female C57L / J wild-type 6-8 week old mice were purchased from Jackson Laboratory (BarHarbor, ME). Mice were housed on a 12:12 h light-dark schedule with ad libitum access to water and food. These experiments were performed in accordance with the Guide to Laboratory Animals for Biomedical Research (Revised 2011) published by the National Institutes of Health. The study protocol was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
[0104] Experimental treatment plan
[0105] Equal numbers of male and female mice were assigned to each group. Mice were subjected to 5 Gy total body irradiation (TBI) using a Varian 21EX LINAC (Palo Alto, CA) under ketamine / xylazine anesthesia (100 mg / kg and 10 mg / kg, respectively), followed by immediate thoracic supplementation 6.5 Gy dose for a total chest dose of 11.5 Gy. Twenty-four hours after irradiatio...
Embodiment 2
[0106] Example 2. Assessment of Cardiac Function
[0107] echocardiography
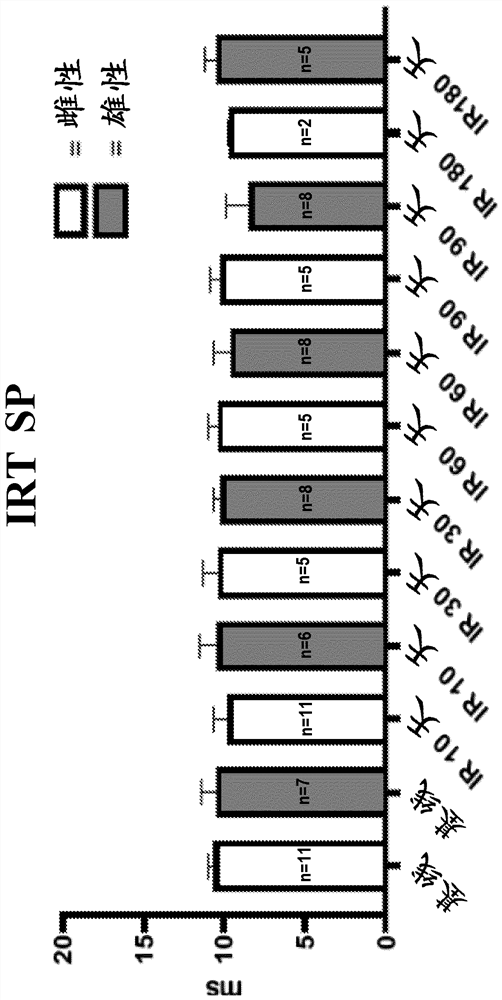
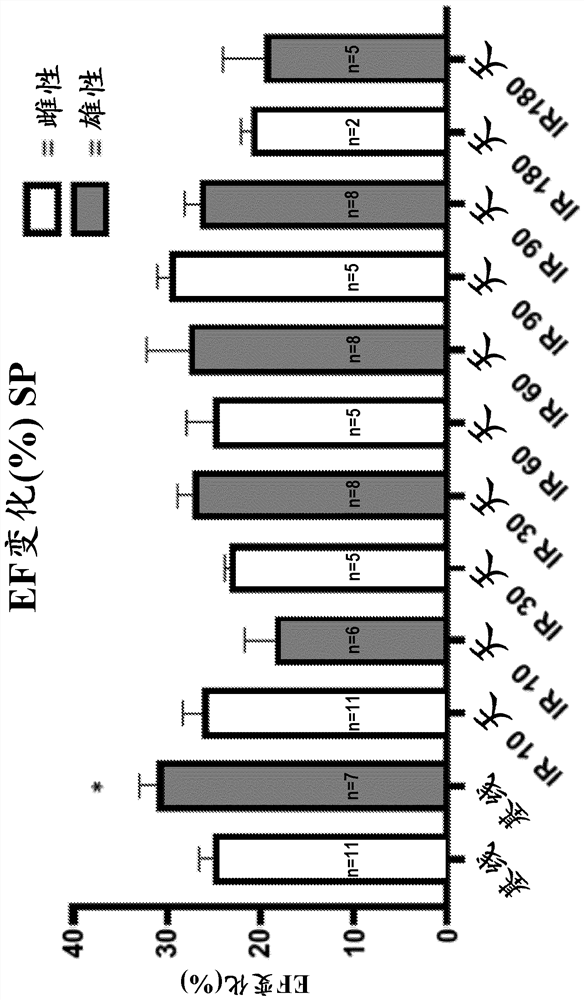
[0108] Under light anesthesia (30 mg / kg sodium pentobarbital), all mice underwent transthoracic echocardiography at baseline (before irradiation (IR)), 8, 30, 60, 90, and 180 days. Echocardiography was performed with a Vevo770 imaging system (VisualSonics, Toronto, Ontario, Canada) and a 30 MHz probe. The heart is shown in B-mode from parasternal short-axis and apical views. Left ventricular (LV) end-diastolic diameter (EDD), LV end-systolic diameter (ESD), LV anterior wall diastolic thickness (AWDT) and LV posterior wall diastolic thickness (PWDT), LV anterior wall systolic thickness (AWST) and Posterior LV systolic thickness (PWST) was measured in M mode according to the recommendations of the American Society of Echocardiography. LV ejection fraction (EF) and LV mass were calculated from measurements of wall thickness and chamber diameter. As mentioned earlier, LVEF is derived using Teicholz'...
Embodiment 3
[0111] Example 3. Assessment of Pulmonary Function
[0112] Assessment of lung injury by respiratory rate
[0113] Beginning at week 6, respiratory rate was measured every other week using the Mouse Ox system (STARR Life Sciences Corp., Allison Park, PA). Animals were placed under ketamine / xylazine anesthesia and shaved in the analysis area. Ten minutes after injection, the animal was placed in the supine position with the transducer clamped to the upper thigh. The respiratory rate was recorded for 3 minutes. Respiratory rate was calculated using an algorithm in the Mouse Ox software.
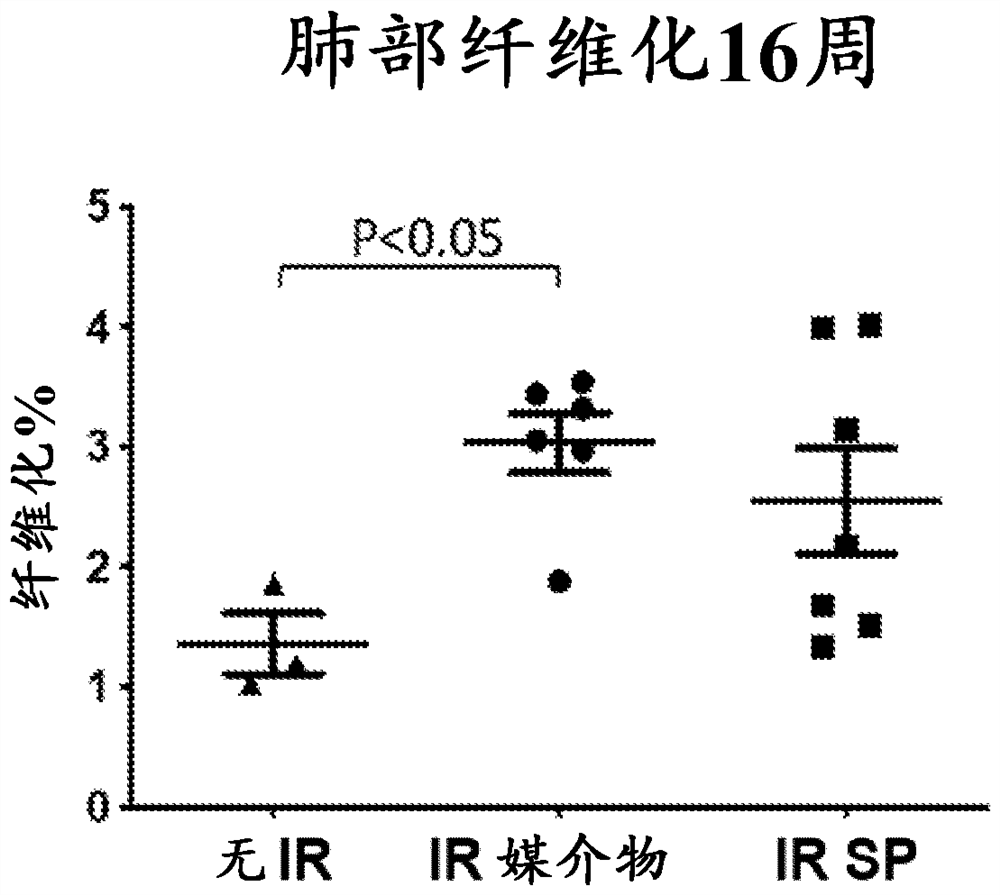
[0114] SP and BH4 reduce radiation-induced lung function loss
[0115] The mouse respiration rate was measured using the MouseOx system as exposure, exposure and SP ( Figure 9 ) and irradiation with BH4 ( Figure 5 ) measure of respiratory function in mice. Mice receiving 1 mg / kg / day SP or 5 mg / kg BH4 for 6 days had a significant delay in the development of lung lesions compared to vehi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap