Anti-CD47 antibodies, activatable anti-CD47 antibodies, and methods of use thereof
A technology of antibodies and antigens, applied in the fields of anti-CD47 antibodies, activatable anti-CD47 antibodies and their use, can solve problems such as limiting the effectiveness of therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0617] The studies presented herein were designed to evaluate the binding of anti-human CD47 antibodies of the present disclosure.
[0618] The anti-human CD47 monoclonal antibodies of the present disclosure were obtained using mouse hybridoma technology according to techniques known in the art. Mice were immunized with human CD47 and hybridomas were screened for binding to human CD47 by ELISA, followed by cytotoxicity in a piggyback assay and cell surface binding by FACS. The mouse anti-human CD47H4L2 monoclonal antibody of the present disclosure includes the heavy chain variable region (VH) of SEQ ID NO:1 and the light chain variable region (VL) of SEQ ID NO:2. In some embodiments of the present disclosure, an exemplary antibody of the present disclosure includes a heavy chain having the H4L2 heavy chain variable region of SEQ ID NO:1 and a light chain having the H4L2 light chain variable region of SEQ ID NO:2.
[0619]Mouse anti-human CD47 monoclonal antibody H4L2 was huma...
Embodiment 2
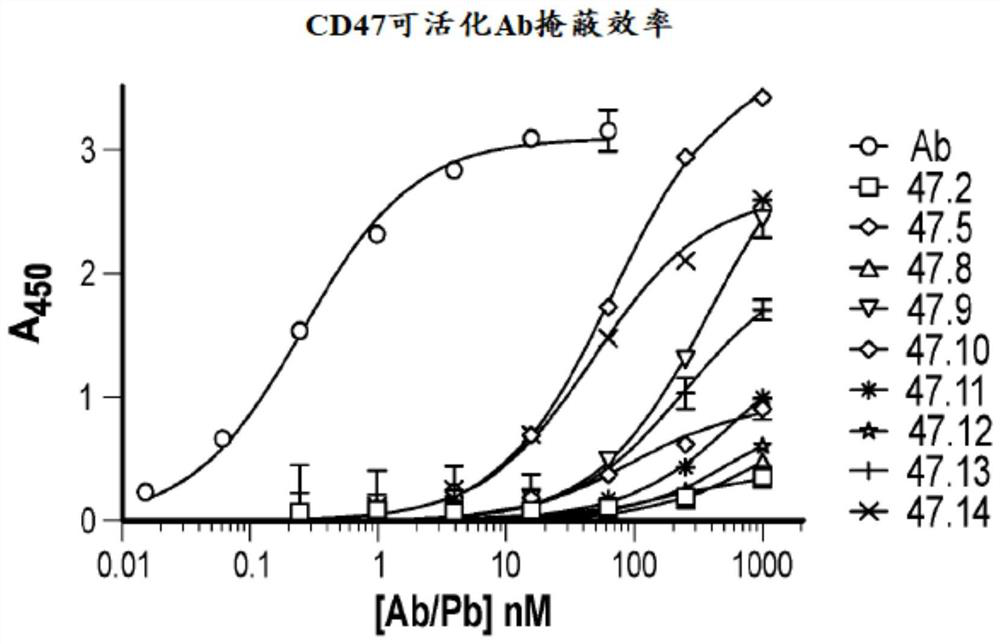
[0661] The studies presented herein were aimed at identifying and characterizing masking moieties for use in the activatable anti-human CD47 antibodies of the present disclosure.
[0662] Using methods similar to those described in PCT International Publication No. WO 2010 / 081173 published on July 15, 2010, the humanized anti-human CD47 hu H4L2 monoclonal antibody (VH and The VL of SEQ ID NO:2) was used to screen for a total diversity of 4x16 0 Random X of 15 A library of peptides, wherein X is any amino acid. Screening included Magnetic Activated Cell Sorting (MACS) and Fluorescence Activated Cell Sorting (FACS). The amino acid sequence of an exemplary single clone of the isolated and sequenced masking peptide (MM) is shown in Table 4.
[0663] Table 4. Anti-human CD47 masking peptide (MM)
[0664]
[0665]
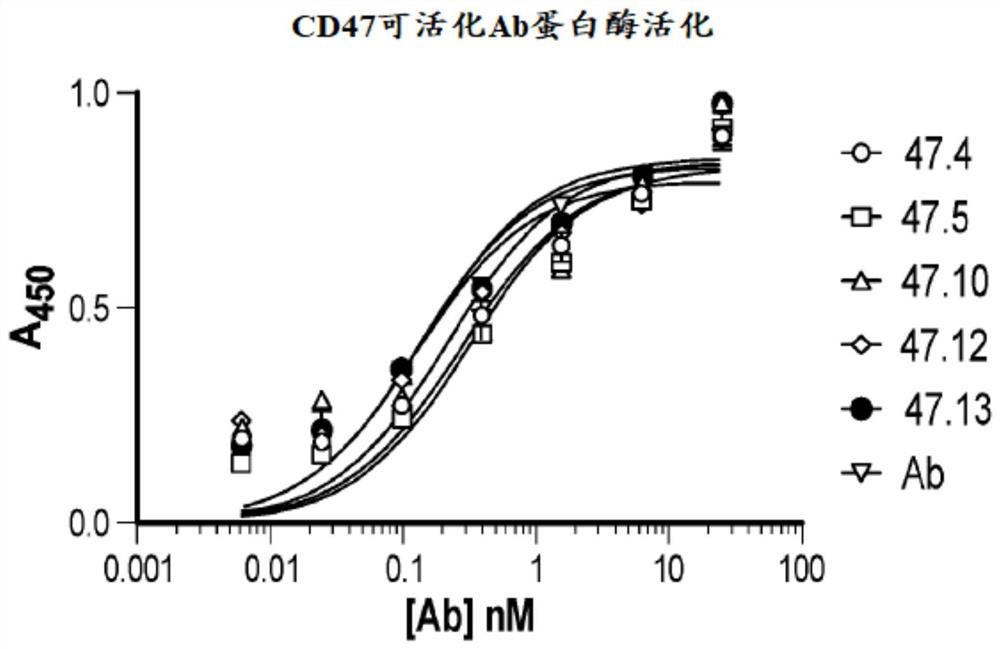
[0666] These masking peptides were used to generate the anti-human CD47 activatable antibodies of the present disclosure. The sequences of some of these ant...
Embodiment 3
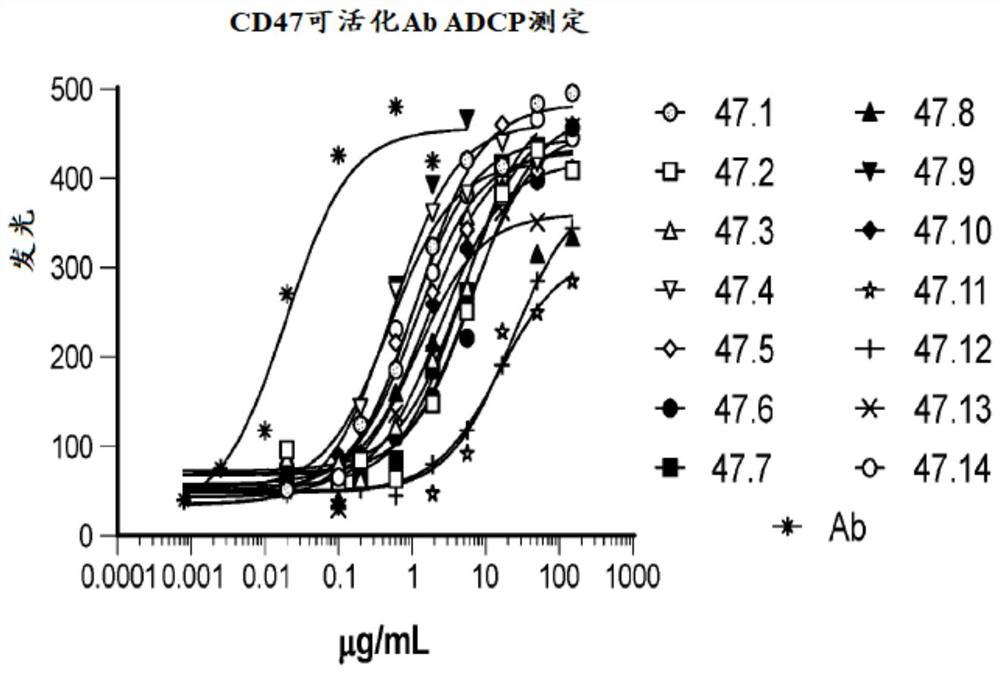
[0730] The studies presented herein were designed to determine that activatable anti-human CD47 antibodies of the present disclosure induce antibody-dependent cellular phagocytosis (ADCP) by affecting the FcyRIIa receptor pathway. As these exemplary results demonstrate, intact activatable antibodies containing masked peptides of the present disclosure exhibit lower ADCP activation through the FcyRIIa receptor pathway compared to unmasked parental anti-CD47 antibodies.
[0731] In this assay (FcyRIIa-H ADCP Reporter Bioassay, Promega), target cells expressing the target antigen are co-cultured with an engineered effector cell line with the FcyRIIa-H receptor. In the presence of an antibody that binds the target antigen through the antigen-binding domain while binding to FcγRIIa on the effector cell through the Fc effector domain, antibody binding results in aggregation of FcγRIIa receptors from the effector cell line, intracellular signaling and NFAT-RE mediation. induced lucif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com