Selection of test vectors for MRI security testing for implantable medical device design
An implantable medical, test vector technology, applied in the field of evaluating the safety of implanted medical device design when it is subject to radio frequency or other interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
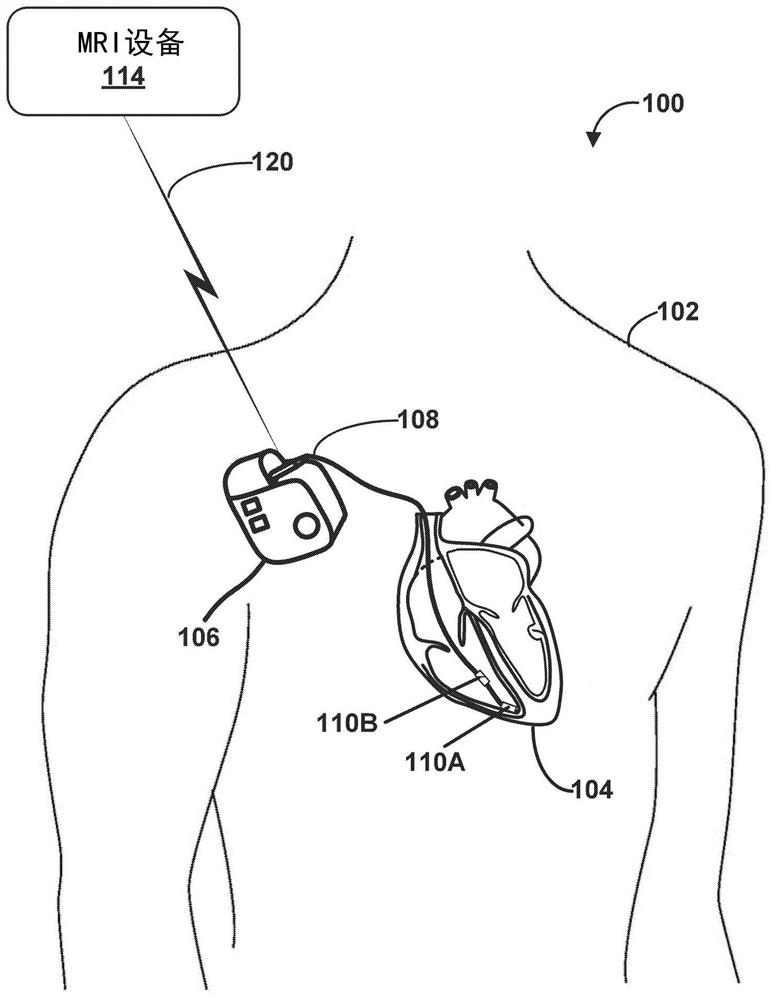
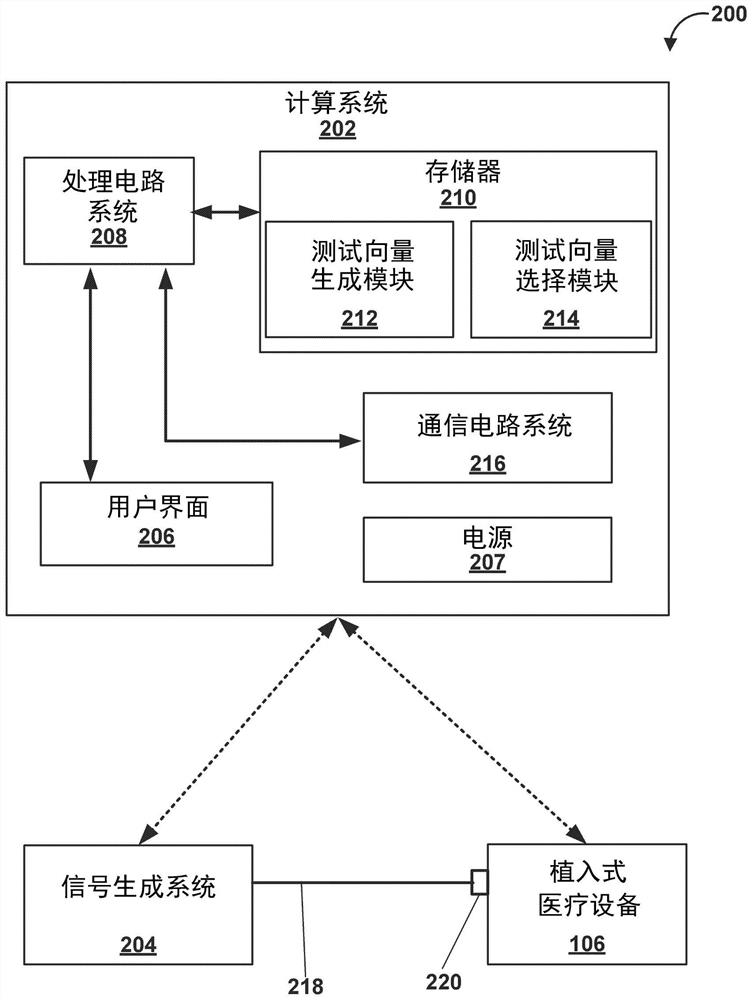
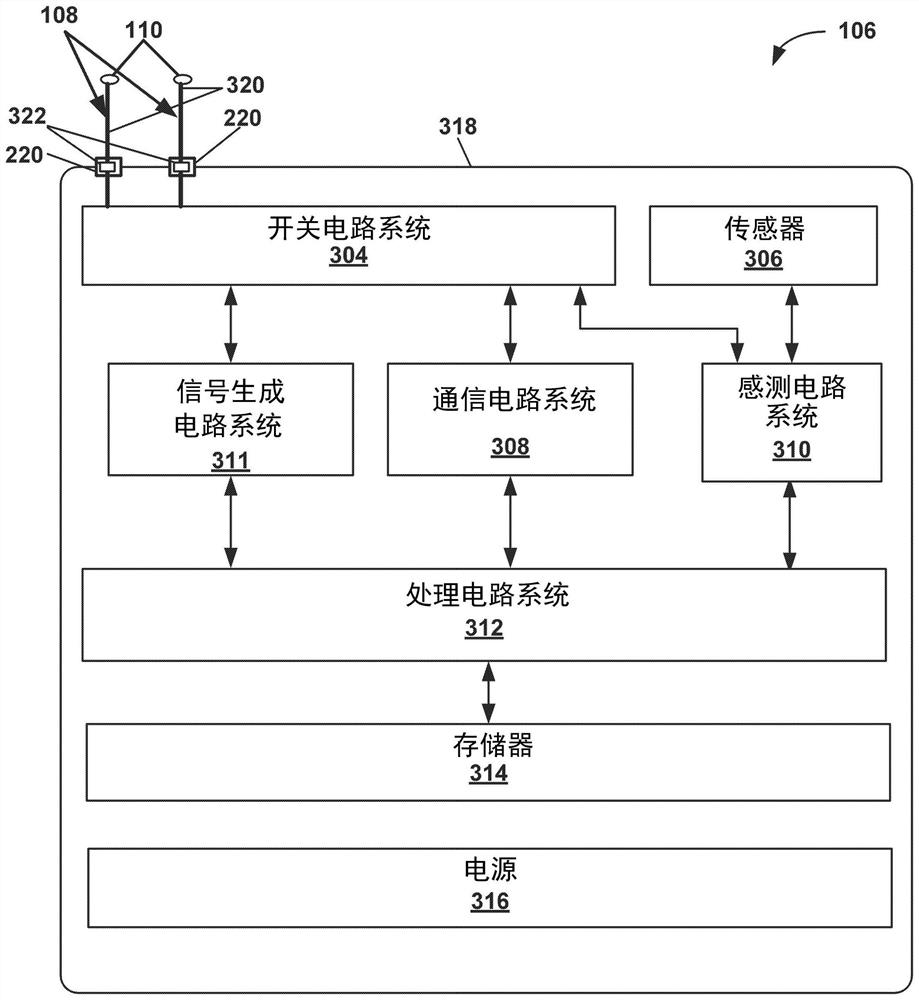
[0075]Example 1: A method comprising: generating, by processing circuitry of a computing system, a plurality of test vectors, each of the plurality of test vectors is a magnetic resonance imaging (MRI) safety test for an implantable medical treatment system A corresponding combination of values of a plurality of parameters, the implantable medical treatment system includes an implantable medical device (IMD) electrically connected to one or more electrical leads, each of the one or more electrical leads comprising at least one electrode on a distal portion of the electrical lead, wherein the plurality of parameters include a patient body model, an MRI scan patient landmark position, a model of one or more electrical leads, one or more The path of each electrical lead within the patient, the type of MRI coil, the type of MRI excitation, and one or more locations of at least one electrode of the one or more electrical leads within the patient; the processing circuitry determine...
example 2
[0076] Example 2: The method of Example 1, wherein generating the plurality of test vectors comprises generating one or more test vectors having a first electrical lead size and a first electrode placement location for the implantable medical device, wherein the first electrical lead The size and first electrode placement maximizes the induced electrical power at the IMD.
example 3
[0077] Example 3: The method of any of Examples 1 and 2, wherein selecting the subset of test vectors from the plurality of test vectors based on electrical power levels and phase values of the subset of test vectors comprises: selecting a first test from the plurality of test vectors vector, wherein the first test vector has the highest electrical power level of any test vector of the plurality of test vectors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com