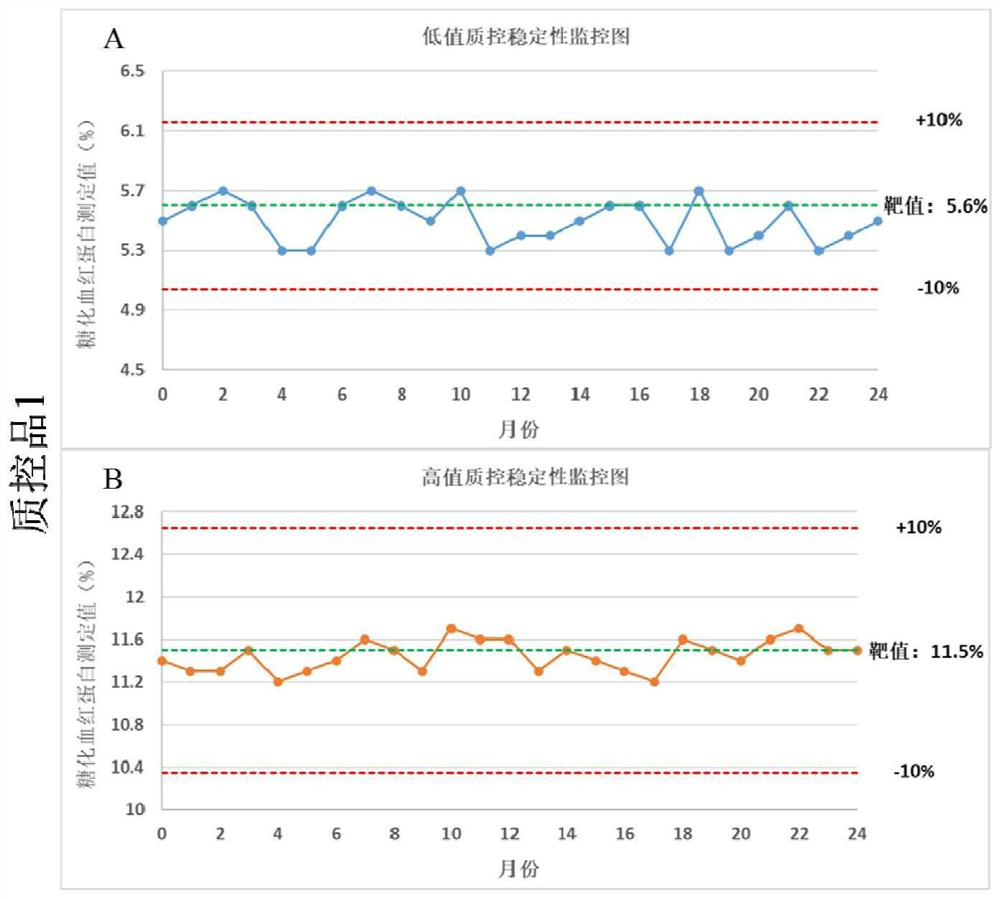
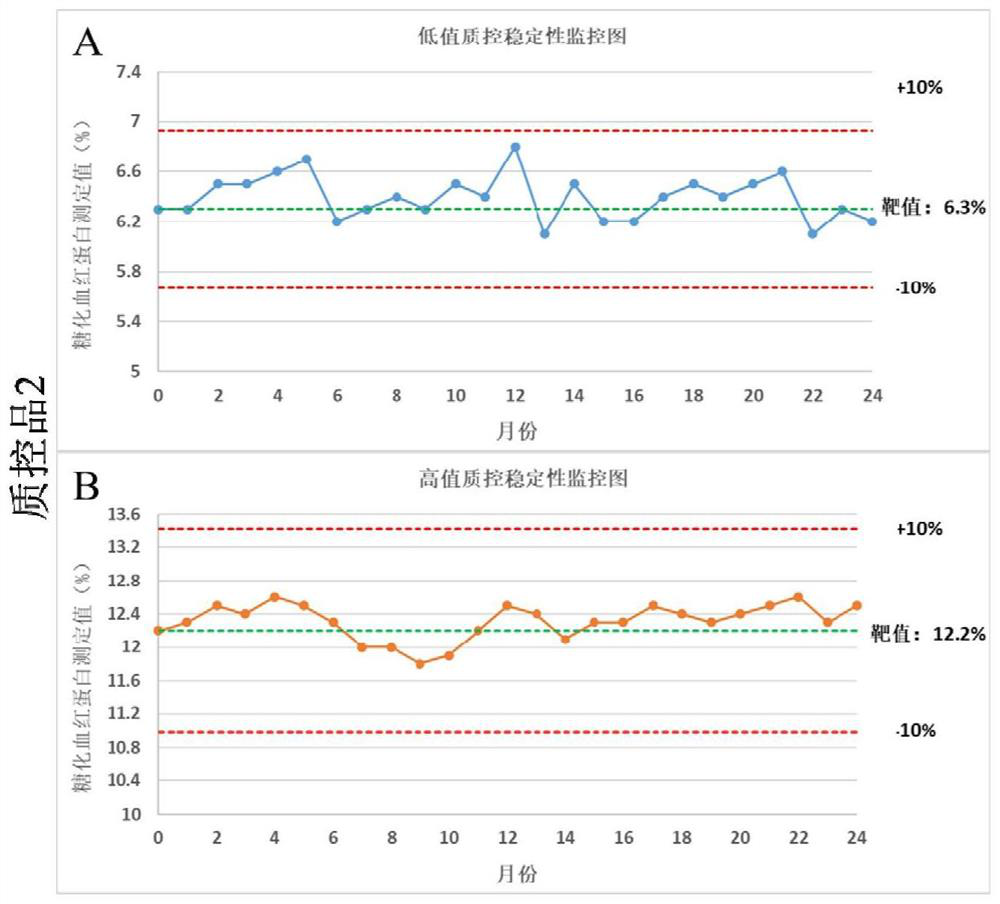
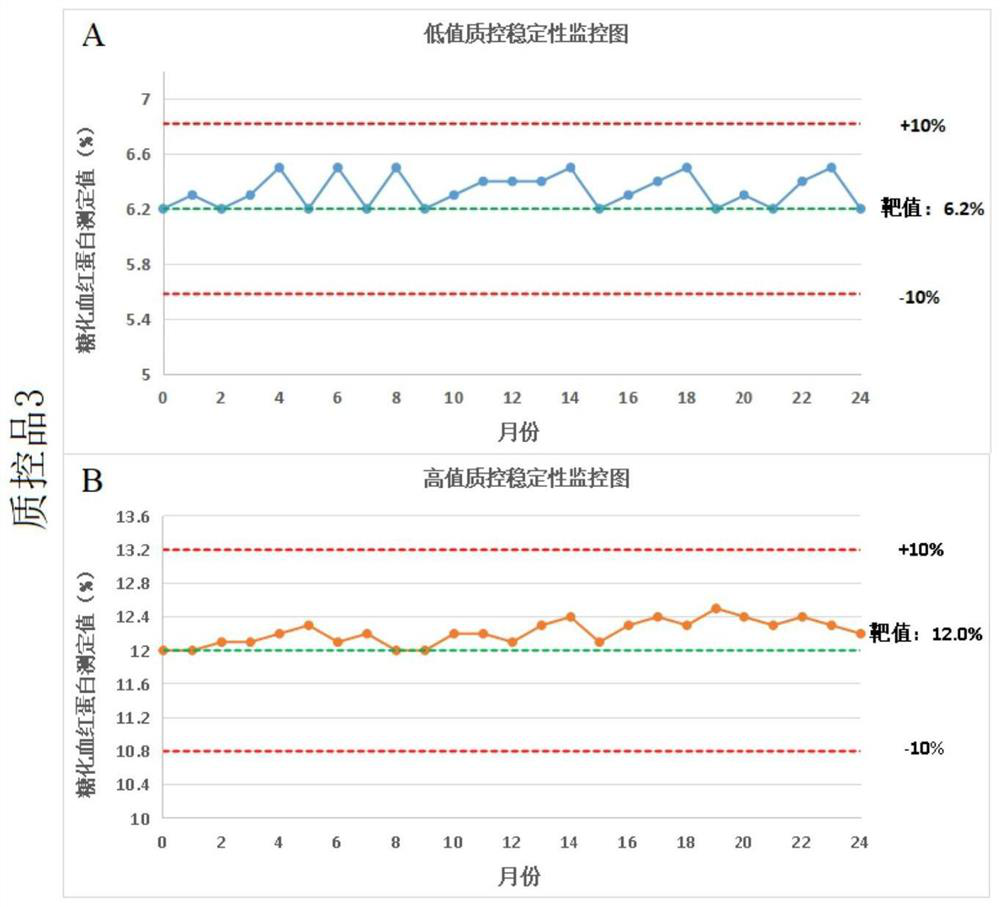
Preparation and application of glycosylated hemoglobin quality control product
A technology of glycosylated hemoglobin and hemoglobin, which is applied in the field of medical testing and determination, and achieves the effects of long storage time, good stability and wide application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of Glycated Hemoglobin Solution 1 Concentrate
[0048] 1. Red blood cell processing
[0049] Collect 200mL of EDTA anticoagulated whole blood samples from healthy people, each sample was tested for HIV (HIV1, HIV2) antibodies, hepatitis B surface antigen (HbsAg) and hepatitis C virus (HCV) antibodies, and the results were all negative. , if one of the items is positive, the samples shall be disposed of in accordance with the relevant medical waste disposal regulations. The collected whole blood samples were centrifuged at about 1900g, the upper plasma was discarded, and normal saline was added, and the red blood cells were washed by pipetting repeatedly with a pipette. .
[0050] 2. Preparation of Glycated Hemoglobin Solution 1 Concentrate
[0051] (1) Preparation of treatment solution
[0052] Take 0.2g of 3-sulfopropyltetradecyldimethylbetaine and 0.028g of sodium nitrite and dissolve them in 100mL of pure water.
[0053] (2) Preparation of ...
Embodiment 2
[0055] Example 2 Preparation of Glycated Hemoglobin Solution 2 Concentrate
[0056] Take the glycated hemoglobin solution 1 (100 mL) prepared in Example 1, add glucose and dissolve it, the final concentration of glucose is 18 mmol / L, and then the mixed solution is placed at 37° C. and incubated for 60 days. After the incubation, glucose oxidase was added to remove unreacted glucose, and the glycosylation reaction was terminated. The final concentration of glucose oxidase was 10KU / L. The solution after glucose removal was placed at 37°C for 7 days to eliminate hydrogen peroxide; Concentrate with an ultrafiltration centrifuge tube with a rate of 30KD, centrifuge at 14000g for 10min to obtain a concentrated solution of glycosylated hemoglobin solution 2 (25mL); store at 2-8°C for later use.
Embodiment 3
[0057] Example 3 Preparation of Glycated Hemoglobin Solution 2 Concentrate
[0058] Take the glycosylated hemoglobin solution 1 (100 mL) prepared in Example 1, add glucose and dissolve it, the final concentration of glucose is 36 mmol / L, and then the mixed solution is placed at 37° C. and incubated for 30 days. After the incubation, glucose oxidase was added to remove unreacted glucose, and the glycosylation reaction was terminated. The final concentration of glucose oxidase was 10KU / L. The solution after glucose removal was placed at 37°C for 7 days to eliminate hydrogen peroxide; Concentrate with an ultrafiltration centrifuge tube with a rate of 30KD, centrifuge at 14000g for 10min to obtain a concentrated solution of glycosylated hemoglobin solution 2 (25mL); store at 2-8°C for later use.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap