Effect of gastric retentive bile acid chelating agent dosage form
A technology of bile acid chelating agent and gastric retention, which is applied in the direction of drug combination, pill delivery, inorganic non-active ingredients, etc., and can solve the problem of incomplete response to PPI treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
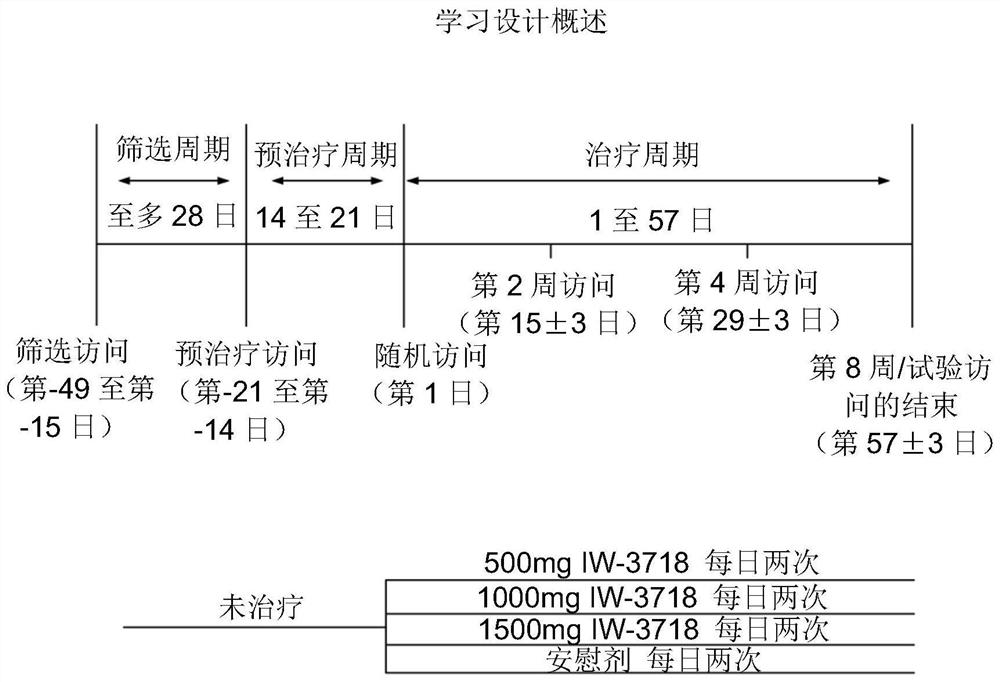
[0186] Example 1: A randomized, double-blind, placebo-controlled, parallel-group, dose-ranging discovery trial to determine oral administration for 8 weeks in symptomatic gastroesophageal reflux disease patients with bile acid sequestering formulations that did not respond completely to proton pump inhibitors safety and efficacy.
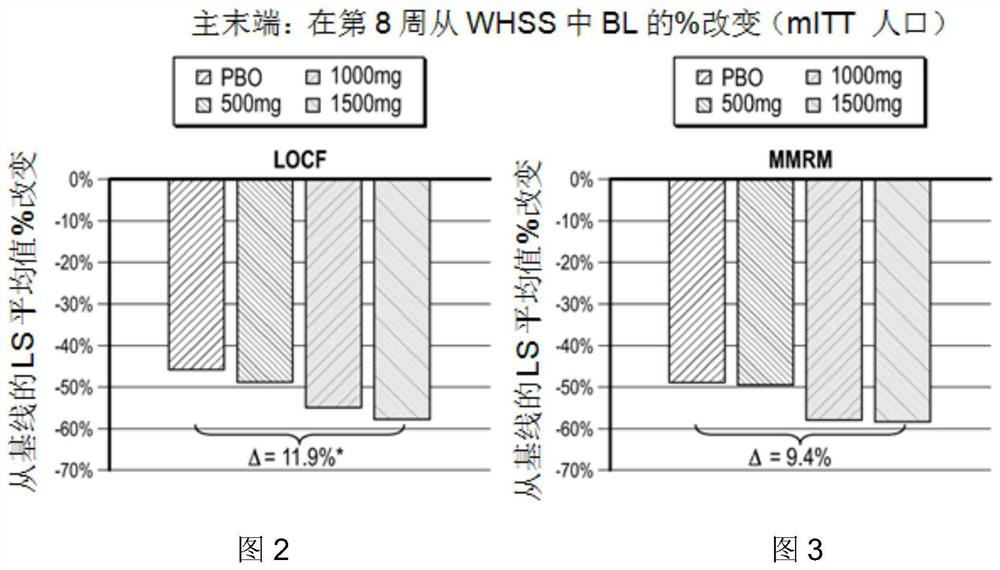
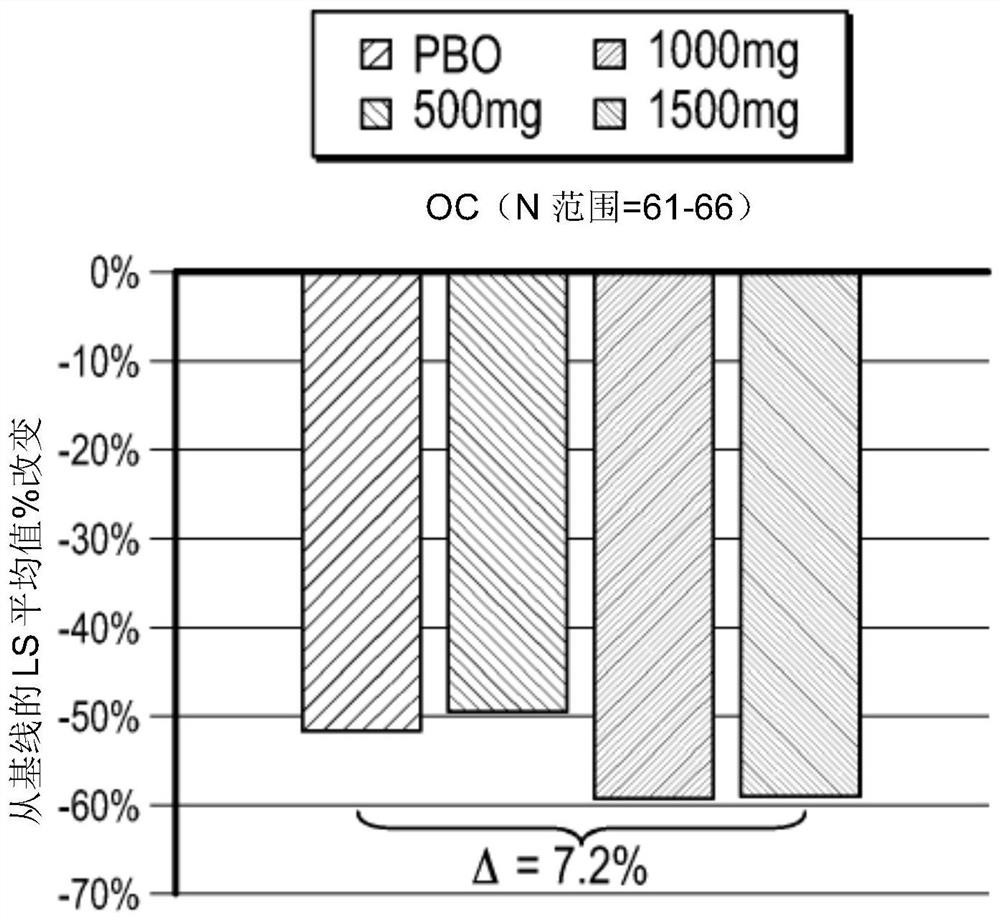
[0187] This case presents the protocol and results of a multicenter (approximately 60-80 centers in the United States), randomized, double-blind, placebo-controlled, parallel-group, 8-week study consisting of 3 distinct periods (screening period up to 28 days; preconditioning period 14-21 days; treatment period 57 days). like figure 1 , the study included patients with gastroesophageal reflux disease who continued to experience gastroesophageal reflux disease symptoms while receiving once-daily treatment with a standard-dose proton pump inhibitor that the investigators believed had been optimized. Eligible patients continued to take proton pump in...
Embodiment 2
[0796]Example 2: Single-center, open-label, randomized, single-dose, 3-lane scintillation imaging study in healthy subjects with 3 periods, each with a difference designed to evaluate the in vivo performance of IW-3718 The in vivo performance of the breakfast composition, IW-3718, was compared to the performance of the immediate release bile acid sequestrant in the fed state.
[0797] The purpose of this study was to compare the gastroprotective properties of a 500 mg tablet of IW-3718 in the fed state compared to an immediate release formulation (comparison product immediate release Cholestagel [bile acid sequestrant; 625 mg]) . The gastrointestinal maintenance properties of the two drugs were studied after breakfast using two drugs with different fat and calorie contents.
[0798] The recommended cholesterol dose is 6 x 625 mg tablets per day; the dose selected in this study was 1 x 625 mg tablet for each of the 3 periods. This dose was well within the recommended daily do...
Embodiment 3
[1107] Example 3, Randomized, Double-Blind, Placebo-Controlled, Parallel Group, Dose-Ranging Exploratory Trial to Determine the Safety and Efficacy of an Oral Bile Acid Sequestrant Dosage Form for Up to 8 Weeks of Administration to Patients with Gastroesophageal Reflux Disease
[1108] Eligibility Criteria
[1109] Inclusion criteria
[1110] Each patient must meet all of the following criteria to be eligible to participate in this study:
[1111] Patients had signed the ICF prior to performing any study-specific procedures.
[1112] Patients were non-hospitalized male or female (if female, not pregnant) and at least 18 years of age at the time of the screening visit.
[1113] Patients had a diagnosis of GERD and reported experiencing GERD symptoms (heartburning or reflux) on average ≥4 days per week during the last 8 weeks prior to the screening visit.
[1114] For at least 8 weeks prior to screening, patients had received standard labeled dose, QD, PPI therapy (which, in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap