Exaltolide preparing process from garlic oil
A technology of cyclopentadecanolactone and garlic fruit oil, applied in the direction of organic chemistry, etc., to achieve the effects of reducing reaction steps, high total yield, and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
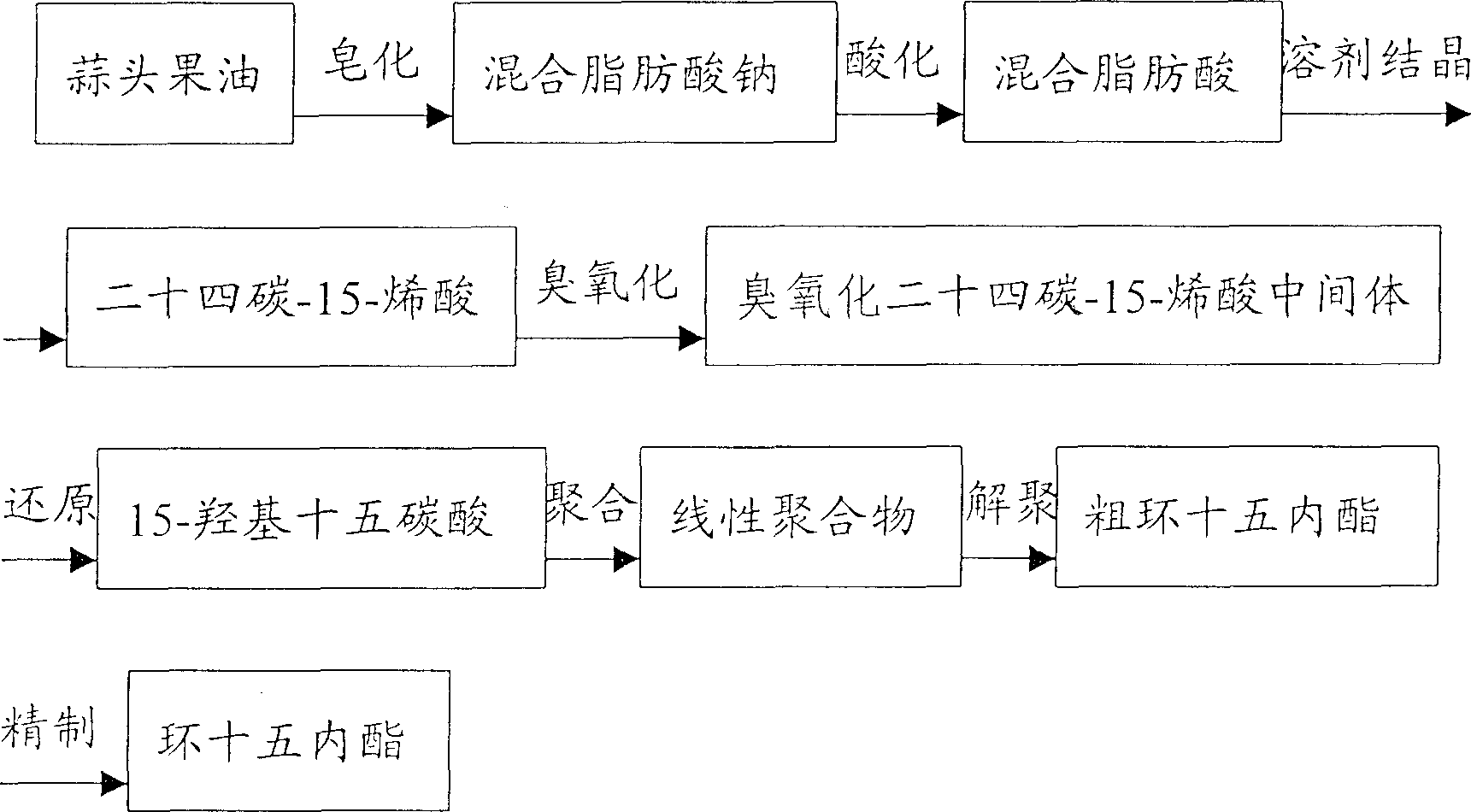
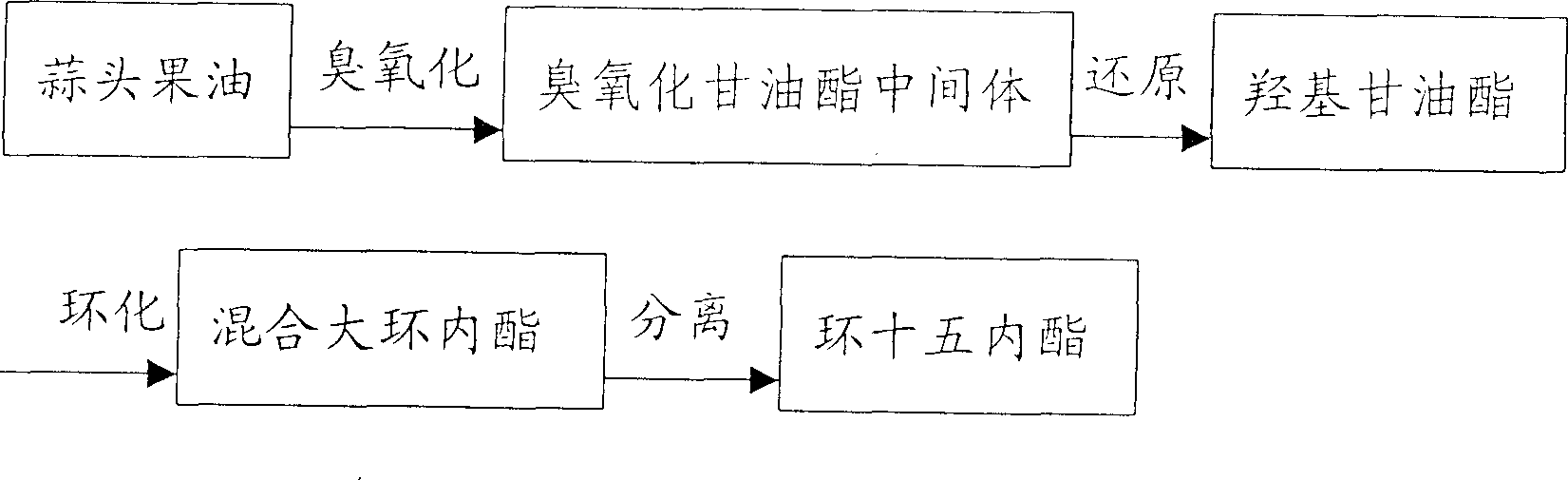
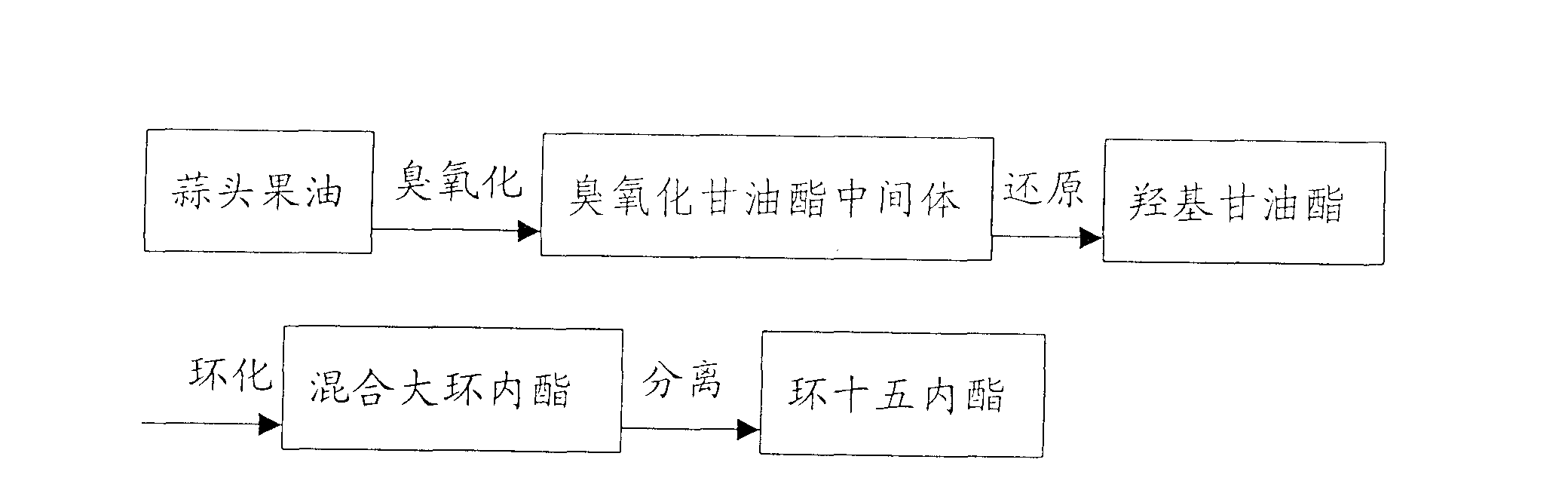
[0016] 2400 grams of garlic fruit oil (about 30% of the mass content of tetracos-15-enoic acid) and 5600 grams of sherwood oil are mixed and put into a gas-liquid reactor, and ozone is fed into the reaction at 20 ° C. When the reaction reaches the end Afterwards, an aqueous solution containing 800 g of potassium borohydride was slowly added dropwise under stirring at 25° C., and the reaction time was 4 hours. Then use sulfuric acid to adjust PH=6~7, stand to separate layers, take the oil layer and heat to evaporate solvent to obtain 2100 grams of hydroxyglycerides. Take 240 grams of hydroxyglycerides and put them into a three-necked flask, add 18 grams of sodium methoxide, heat the reaction solution to 180-230° C. under a vacuum of 3-10 mmHg, and react for 12 hours to obtain 188 grams of the product. After rectification and ethanol crystallization, 48 grams of cyclopentadecanolide with a purity of 98.5% were obtained. The total yield was (2100 / 2400) x (48 / 240) = 17.5%.
Embodiment 2
[0018] 300 grams of garlic fruit oil (about 50% of the mass content of tetracos-15-enoic acid) is mixed with 1000 grams of sherwood oil and 500 grams of ethanol and put into a gas-liquid reactor, and then feed ozone at 10°C for reaction. After the reaction reaches the end point, an aqueous solution containing 65 g of potassium borohydride is slowly added dropwise under stirring at 25° C., and the reaction time is 4 hours. Then use sulfuric acid to adjust PH=6~7, let stand to separate layers, take the oil layer and heat to evaporate solvent to obtain 265 grams of hydroxyglycerides. Take 180 grams of hydroxyglycerides and put them into the reactor, add 7 grams of sodium methoxide, heat the reaction solution to 180-230° C. under a vacuum of 3-10 mmHg, add glycerin as an entrainer for entrainment distillation, and react for 12 hours to obtain the product 130 gram. After rectification and ethanol crystallization, 59 grams of cyclopentadecanolide with a purity of 98.5% were obtaine...
Embodiment 3
[0020] The manufacture method of hydroxyglyceride is with embodiment 2. Take 180 grams of hydroxyglycerides into the reactor, add 9 grams of sodium methoxide, heat the reaction solution to 180-230 ° C under a vacuum of 3-10 mmHg, add glycerin as an entrainer for entrainment distillation, and react for 16 hours to obtain 136 grams of the product. After rectification and ethanol crystallization, 61 grams of cyclopentadecanolide with a purity of 98.5 were obtained. The total yield was (61 / 180)=33.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com