Preparation method of cholera toxin B sub unit
A technology of cholera toxin and subunit, which is applied in the field of producing CTB to prepare drugs, and can solve the problems of cumbersome multi-step operation, low expression and high production cost in the post-processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Construction of CTB gene expression plasmid in Escherichia coli
[0024] Design primers based on the published CTB gene sequence ((Nature, 1983, 306:551-557), introduce the BamHI restriction site at the 5'end of the primer, and introduce the targeting sequence and EcoRI site at the 3'end. Upstream primer It is: 5'GGGGATCCATGATTAAATTAAAATTTGGT3', the downstream primer is: 5'GGGGAATTCTTATAGCTCATCTTTCTCAGAATTTGCCATACTAATTGCGGCAA3', take 1ml of cholera bacteria (Vibrio cholerae), centrifuge at 12000rpm for 5 minutes, take the precipitate, add 5μl 20g / L proteinase K and 10% GIBSOBDS company) ℃, 1 hour, add 500μl phenol, 12000rpm centrifugation for 10 minutes, take the supernatant, add 500μl chloroform (Zhejiang Dier Pharmaceutical Co., Ltd.), centrifuge at 12000rpm for 10 minutes, take the supernatant, add 2 times the volume of ethanol, and mix well Centrifuge at 12000rpm for 10 minutes, discard the supernatant, and add 50μl of water to dissolve. PCR amplification of ...
Embodiment 2
[0025] Example 2. Construction of insect baculovirus transfer plasmid of CTB gene
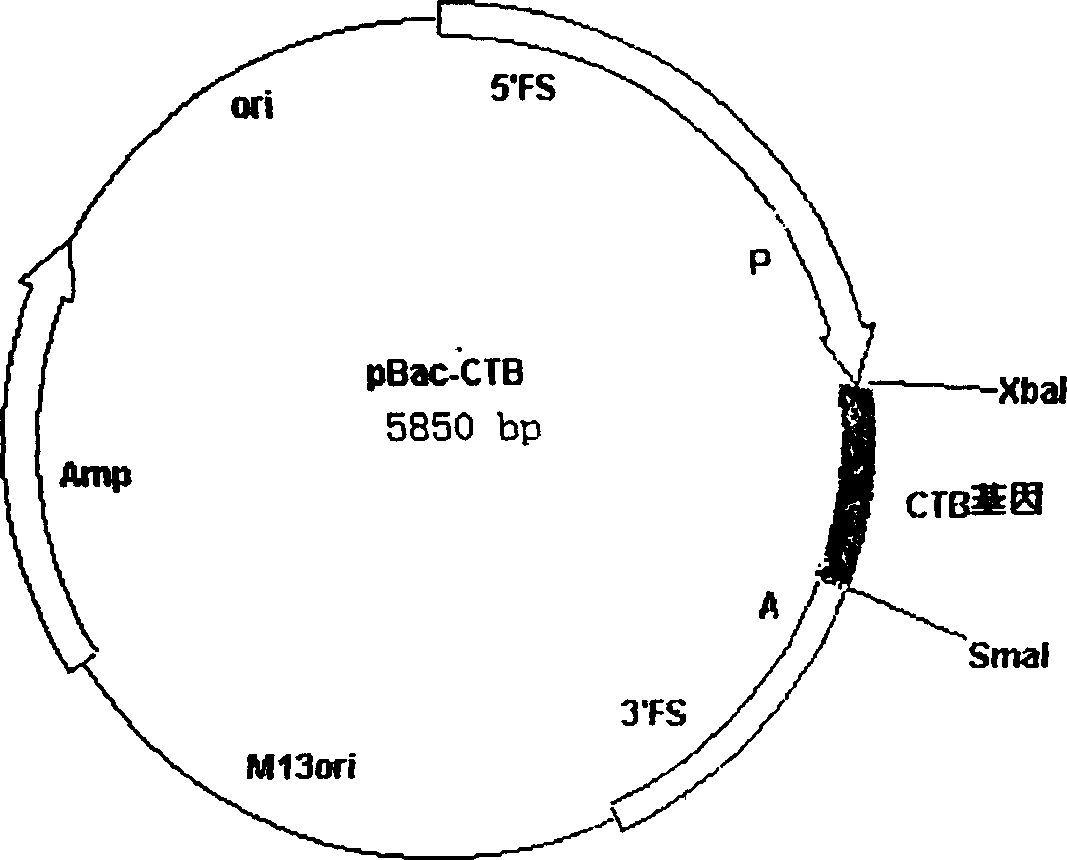
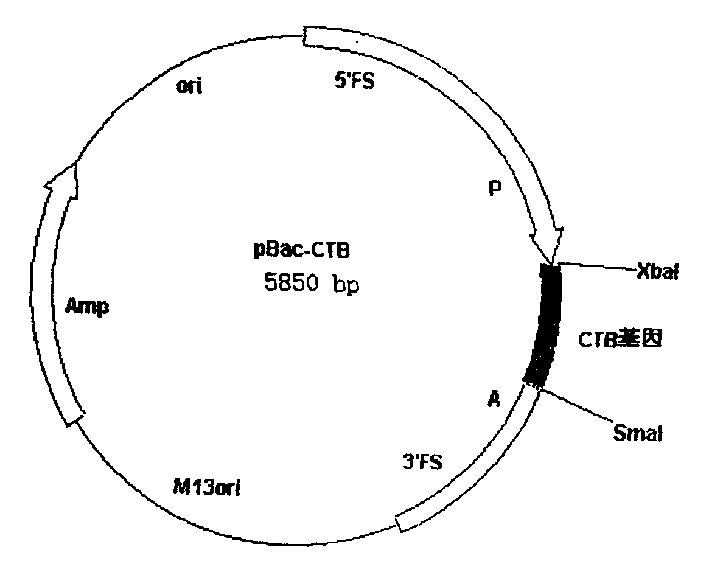
[0026] The pUC-CTB plasmid was digested with BamHI and EcoRI, and the digested fragments were connected to pBacPAK8 (CLONTECH), which was also digested with BamHI and EcoRI, to construct a baculovirus transfer plasmid pBac-CTB ( figure 1 ), the gene was identified as correct by restriction analysis.
Embodiment 3
[0027] Example 3 Obtaining the recombinant baculovirus of CTB gene
[0028] Take 5ul insect baculovirus transfer plasmid pBac-CTB containing CTB gene and 6ul wild Bombyx mori nuclear polyhedrosis virus DNA for co-transfection. Take 6ul Lipofectin (GIBCOBRL company) and add 100ul serum-free TC-100 medium and mix well. Wash the BmN cells pre-cultured in 35mm Dish with serum-free TC-100 (GIBCOBRL company) medium twice, and add the transfer plasmid and Lipofectin mixture dropwise, culture at 27°C for 4-5 days, collect the supernatant and proceed to the first A round of plaque screening. Take 5ul of supernatant to infect BmN cells in 35mmDish, discard the supernatant after 1 hour and add an equal amount of mixed TC-100 medium and low melting agarose. After 4-5 days, the plaques were picked, Bm N cells were infected for 3-4 days, the supernatant was preserved, the cells were lysed with NaOH for Southern hybridization, and the supernatant of positive clones was taken for the 10th round o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com