Fusion protein with cold proventing and curing function and its encoding gene and use
A technology of fusion protein and coding gene, which is applied in the field of fusion protein and its coding gene and vaccines with the protein as the active ingredient, which can solve the problem of unguaranteed immune effect, inability to guarantee the number of M2e epitope polypeptides, and affecting the production and promotion of influenza vaccines and other issues to achieve the effect of improved safety and mature production technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
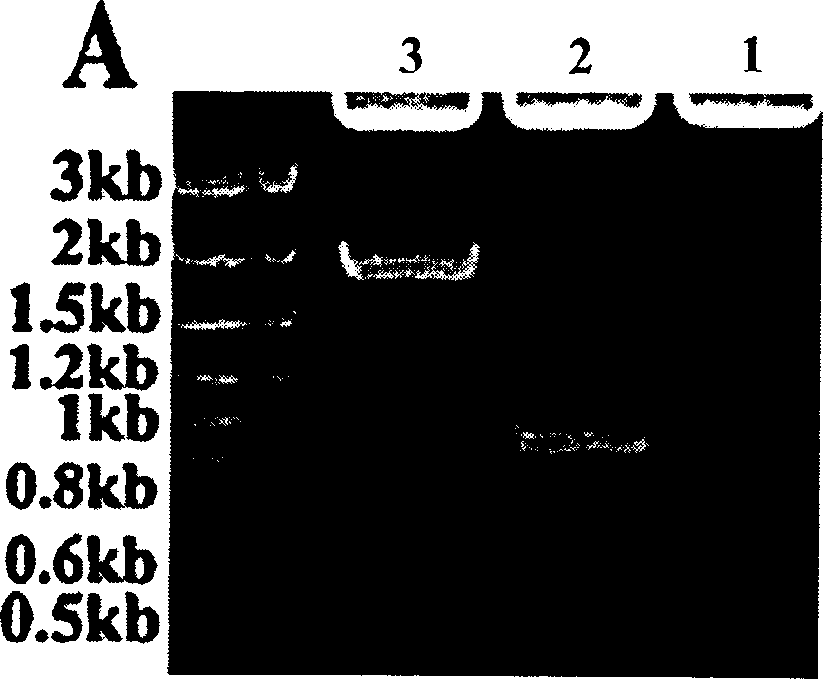
Image
Examples
Embodiment 1
[0032] Embodiment 1, GST-(M2e) 16 , GST-(M2e) 4 and GST-M2e preparation and identification
[0033] 1. GST-(M2e) 16 , GST-(M2e) 4 Construction of three fusion protein genes with GST-M2e
[0034] Oligonucleotide chains L1, L2, P1 and P2 were synthesized by Beijing Aoke Biotechnology Co., Ltd. using 8909 Expedite automatic nucleic acid synthesis instrument, and identified by gel purification. Their sequence is as follows: L1:5`GCGC GGATCC ATGTCCCTGCTGACTGAAGTTGAAACTCCGATCCGTAACGAATG 3' (bases underlined are BamH I recognition sequences);
[0035] L2: 5`GTTATCA AGATCT GTCGGAGGAGTCGTTGCAACGGCAACCCCATTCGTTACGG 3' (the underlined base is the Bgl II recognition sequence);
[0036] P1: 5`GTGCGC GGATCC 3' (the underlined base is the BamH I recognition sequence);
[0037] P2: 5` CTCGAG TTATCA AGATCT 3' (the underlined base at the 5' end is the Xho I recognition sequence, and the underlined base at the 3' end is the Bgl II recognition sequence)
[0038] Use P1 and P2 as ...
Embodiment 2
[0058] Embodiment 2, GST-(M2e) 16 Induced antibody level experiments
[0059] image 3 Graphically represent GST, GST-M2e, GST-(M2e) with legend 4 and GST-(M2e) 16 The epitope density of M2e in M2e, using M0, M1, M4 and M16 to represent GST, GST-M2e, GST-(M2e) respectively 4 and GST-(M2e) 16 .
[0060] Carry out the immunization experiment of mouse (BALB / c) and New Zealand white rabbit as animal model according to the following method:
[0061] A total of 32 female Balb / c mice of 16-20 grams were randomly divided into four groups, and immunogens (GST, GST-M2e, GST-(M2e) 4 or GST-(M2e) 16 ) to immunize 8 mice intraperitoneally. For the first time, each mouse was injected with complete Freund's adjuvant (PIERCE company product) 1:1 emulsified recombinant immunogen diluted in PBS 50 μg, and the immune volume was 200 μl for the first subcutaneous multi-point (4 points) injection immunization, and each point was injected subcutaneously. 50 μL. Afterwards, they were immuni...
Embodiment 3
[0064] Embodiment 3, GST-M2e, GST-(M2e) 4 and GST-(M2e) 16 The concentration of induced rabbit serum M2e-specific antibody and the experiment of recognizing the natural M2 protein of influenza virus
[0065] 1. Identification of GST-(M2e) using M2e epitope peptide-specific affinity chromatography column 16 , GST-(M2e) 4 , The principle and steps of the preparation of the experimental affinity chromatography column for the concentration of the M2e epitope polypeptide-specific antibody in the rabbit antiserum induced by GST-M2e: using the active group NHS-easy on the column product Sepharose4-FAST of Pharmacia Company NH with M2e epitope peptide amino acid 2 - The characteristics of group combination reacting and relatively stable coupling complete the preparation of the affinity chromatography column.
[0066] (1) Draw 1ml of NHS-activated_Sepharose4-FAST column material (Pharmacia company), and 2mg of M2e epitope polypeptide (synthesized by American Genemed Synthesis compa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap