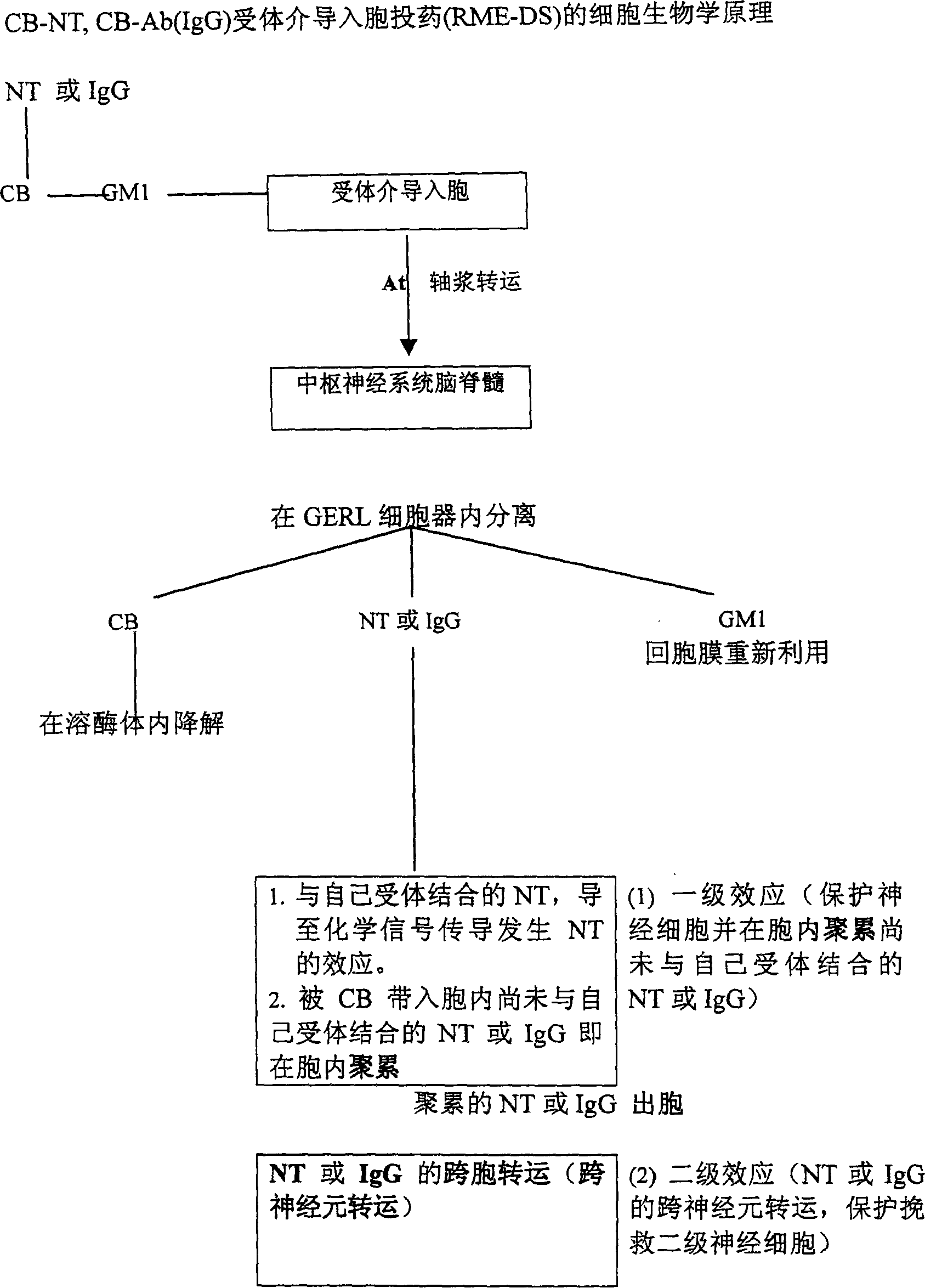
Coupling agent of CB and specific immune globulin IgG and use thereof
A technology of conjugates and therapeutic effects, applied in the field of conjugates of CB and specific immunoglobulin IgG and its medical application, can solve the unobserved CB-GM1 receptor-mediated intracellular or nervous system diseases and effects Problems with brain function and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0063] Glutaraldehyde GA Conjugation of CB-IgG [Rabbit Anti-Substance P]
[0064] CB [pentamer pentamer molecular weight 45-50 kDa, List Ltd., US] 3.0 mg dissolved in 1.25% GA [Sigma, used for electron microscope specimens] 1 ml, 0.1 M sodium phosphate (pH 6.5) at room temperature overnight [Compared to Low pH prevents the self-coupling of the amino group of lysine in CB]; activated CB and unlinked glutaraldehyde were separated by Sephadex G-25 column (40×0.9cm, with 0.15M NaCl solution passed through the column) (or do not use the column and dialyze against 0.15M NaCl to remove unlinked glutaraldehyde); use a microporous membrane to concentrate the CB fraction (molecular weight 45-50kDa) of the column solution to about 1ml.
[0065] Adjust the pH of the concentrated activated CB to 9.5 with 1M carbonate buffer (pH 9.5), add rabbit anti-substance P IgG (molecular weight 150kDa) 9.0mg dissolved in 1M carbonate buffer (pH9.5) 1ml, Act with activated CB at a low temperature of 4...
preparation Embodiment 2
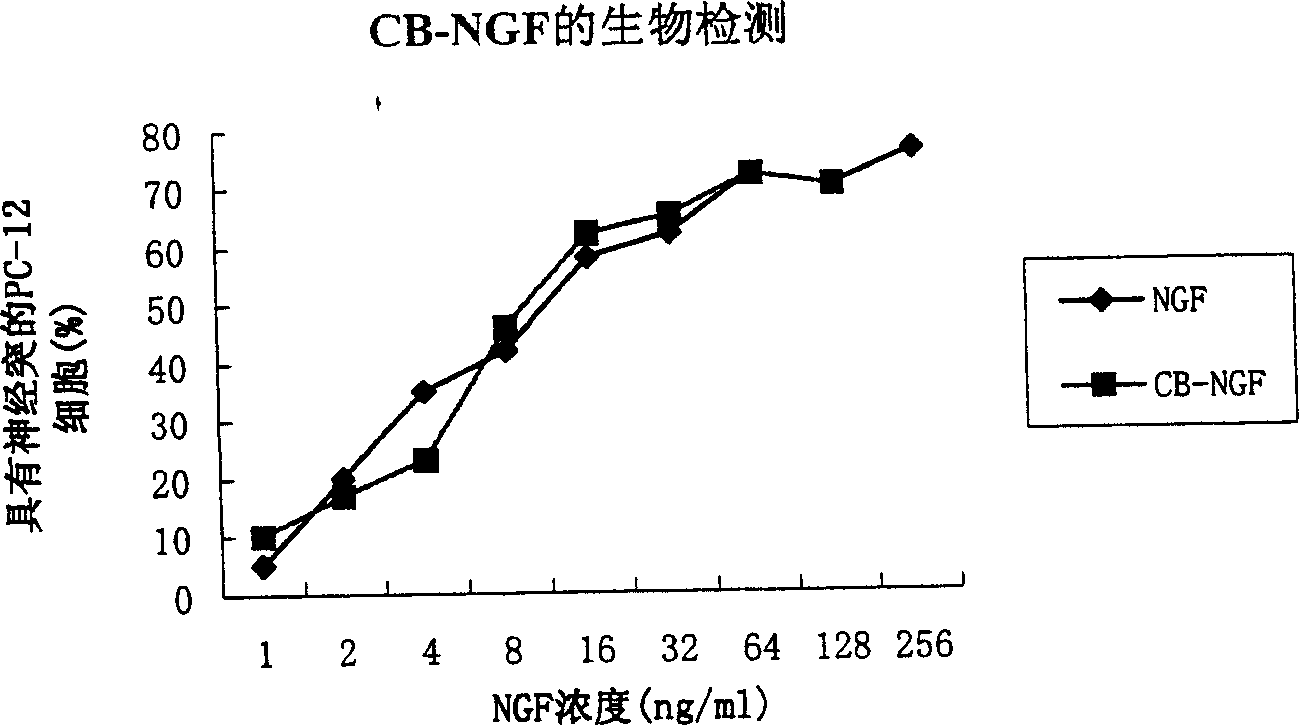
[0067] GA coupling of CB-NGF
[0068] CB [molecular weight 45-50kDa, List Ltd., US] 4mg dissolved in 1.25% GA [Sigma, for electron microscope specimens] 1ml, 0.1M sodium phosphate (pH6.5) at room temperature overnight [lower pH can prevent Amino group self-coupling of lysine in CB]; activated CB was separated from unlinked glutaraldehyde (or non-column but Dialysis against 0.15M NaCl to remove unconnected glutaraldehyde); the CB fraction (molecular weight 45-50kDa) passed through the column solution was concentrated to about 1ml with a microporous membrane.
[0069] The pH of the concentrated activated CB was adjusted to 9.5 with 1 M carbonate buffer (pH 9.5), and NGF (betaNGF, 4 mg two-chain, molecular weight 26.0 kDa, Sigma, N 8133) 2.0 mg dissolved in 1 M carbonate was added Buffer solution (pH9.5) 1ml, with activated CB at 4 degrees low temperature for 24 hours; Sephadex G-200 gel column (1.6×90cm) separates bound (molecular weight 80kDa) and unbound components; Concentra...
preparation Embodiment 3
[0071] Disulfide bond S-S coupling process of CB-NGF
[0072]Dissolve 2.0 mg of NGF (beta NGF, two chains, molecular weight 26.0 kDa, Sigma, N8133) in 1 ml, 1 M carbonate buffer (pH 8.5), and slowly add 6.0 mg / ml citraconic anhydride (citric anhydride). , Sigma, US), placed at room temperature for 1 hour to block free amino groups; G10 gel column was used to separate citric anhydride and NGF peptide. Add 20mg of EDC to 2.0mg / 2ml of NGF to act on the carboxyl group, first adjust the pH to 5, then adjust to 8, and leave it at room temperature for 10 minutes; remove EDC through G10 gel column; concentrate NGF solution to 2ml; dropwise add 20mM PDP solution ( Sigma, US) 0.5ml in NGF solution, stirred for 30 minutes at room temperature; excess PDP was removed through Sephadex G-25 (0.1M, PBS elute) gel column, and the NGF solution was concentrated to 2ml by microporous membrane.
[0073] 4 mg of CB (List, US) was dissolved in 1 ml of 1 M carbonate buffer (pH 8.5), 0.5 ml of 20 mM ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap