Method for making hydroxy-25-ene-vitamin D compounds
A technology for vitamins and compounds, applied in the field of preparing hydroxy-25-ene-vitamin D compounds and vitamin D compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
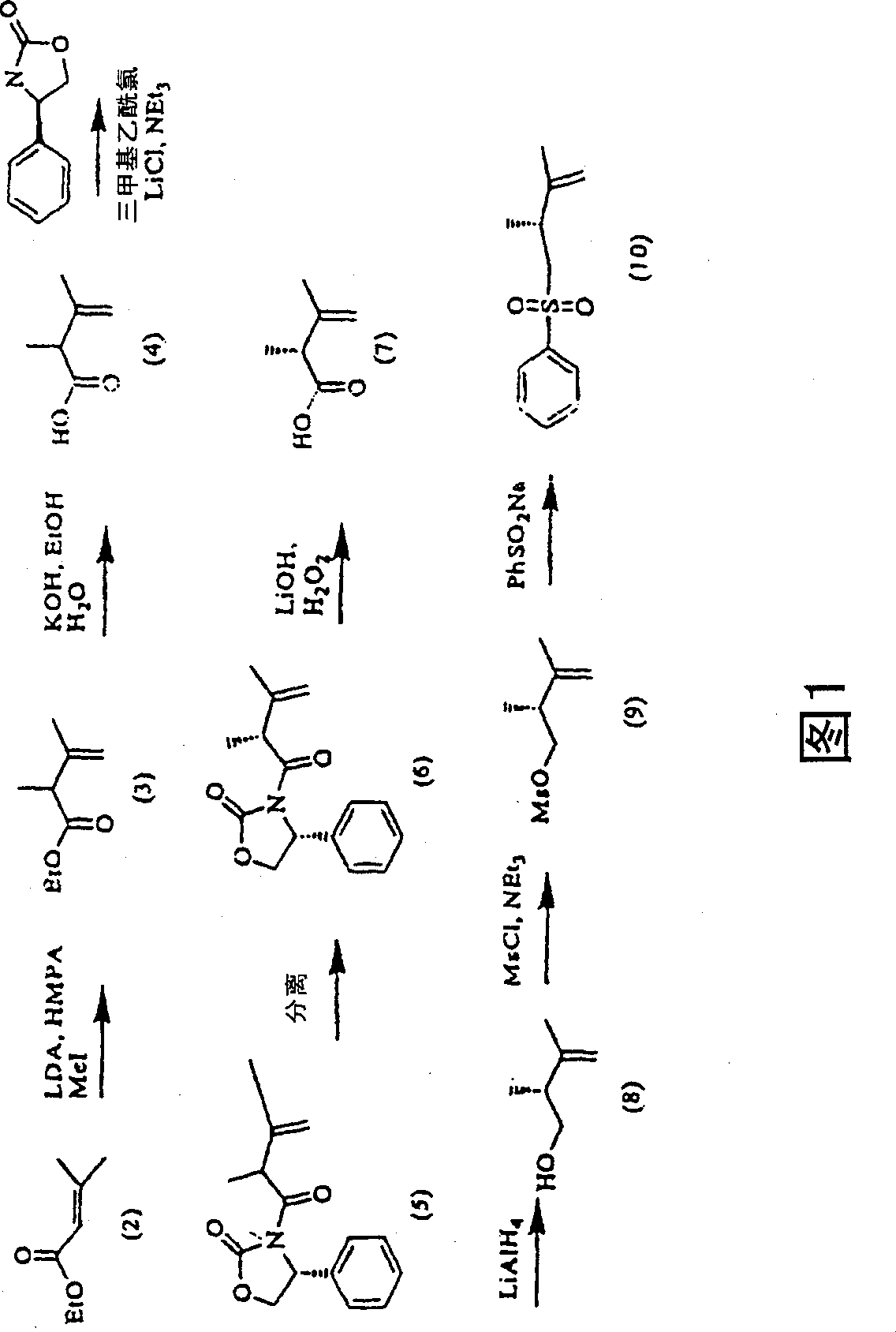
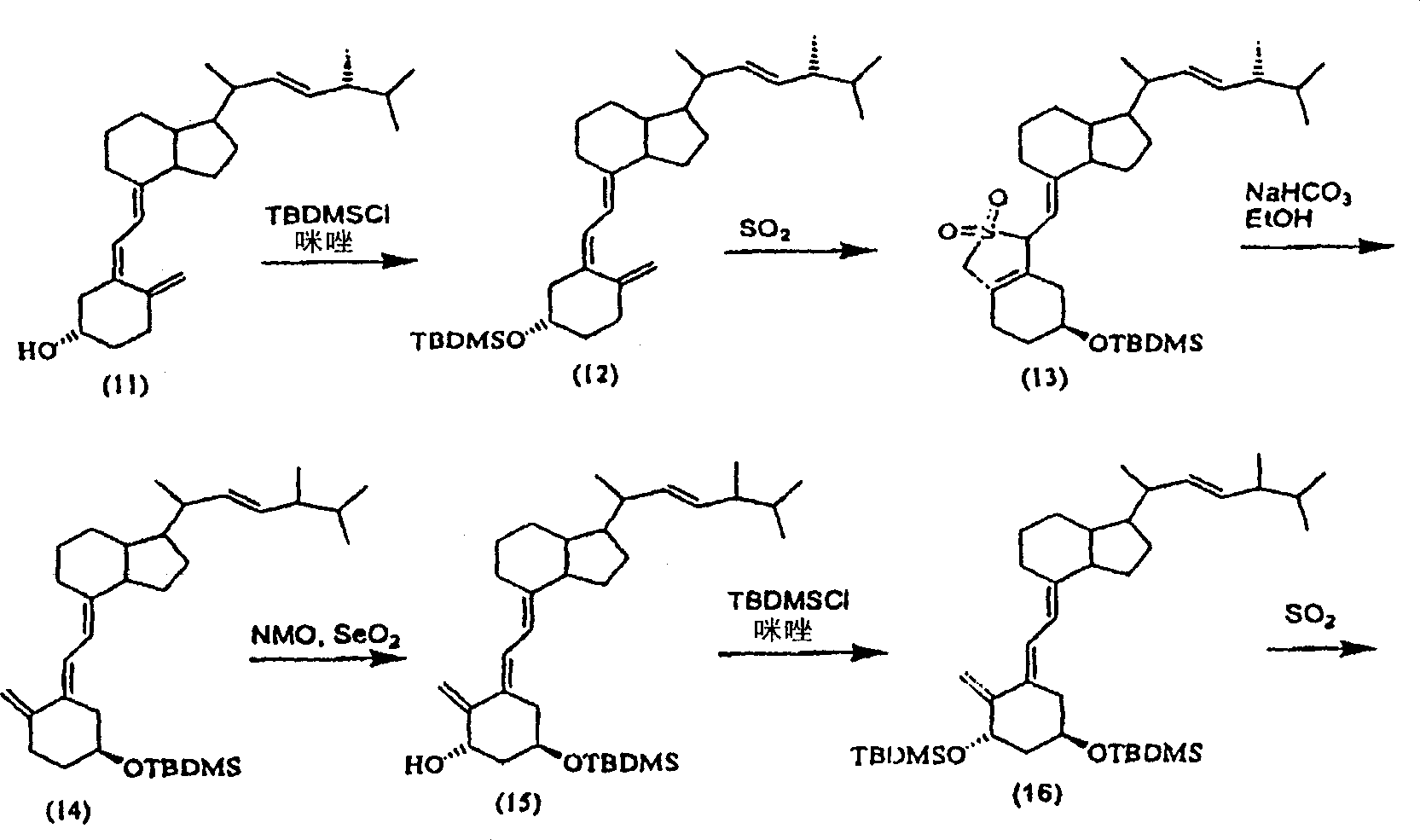
[0075] 1 H-NMR spectra were recorded by Varian VXR-300. Chemical shifts δ (ppm) are marked with reference to TMS. For HPLC analysis, a platinum EPS C18 150×4.6mm column was used, and the mobile phase was CH 3CN-0.1% COOH 70:30, detection wavelength 265nm, flow rate 1ml / min, temperature 22°C. Melting points were determined on a Mettler FP-2 melting point apparatus equipped with a Mettler FP21 microscope. Example 1: Synthesis of 1α-hydroxyl-25-ene-vitamin D Preparation of R-(2,3-dimethyl-3-buten-1-yl) phenyl sulfone (10)
[0076] To a solution of 110ml of diisopropylamine in 750ml of tetrahydrofuran (THF), 315ml of 2.5N n-butyllithium (n-BuLi) was added, and the reaction temperature was between -25°C and -10°C. The mixture was cooled to -70°C, then 150 ml of HMPA was added dropwise, and the reaction mixture was stirred for an additional hour at this temperature. After the solution of 100 g of ethyl dimethacrylate (2) in 100 ml of THF was added dropwise, the reaction mixture...
Embodiment 2
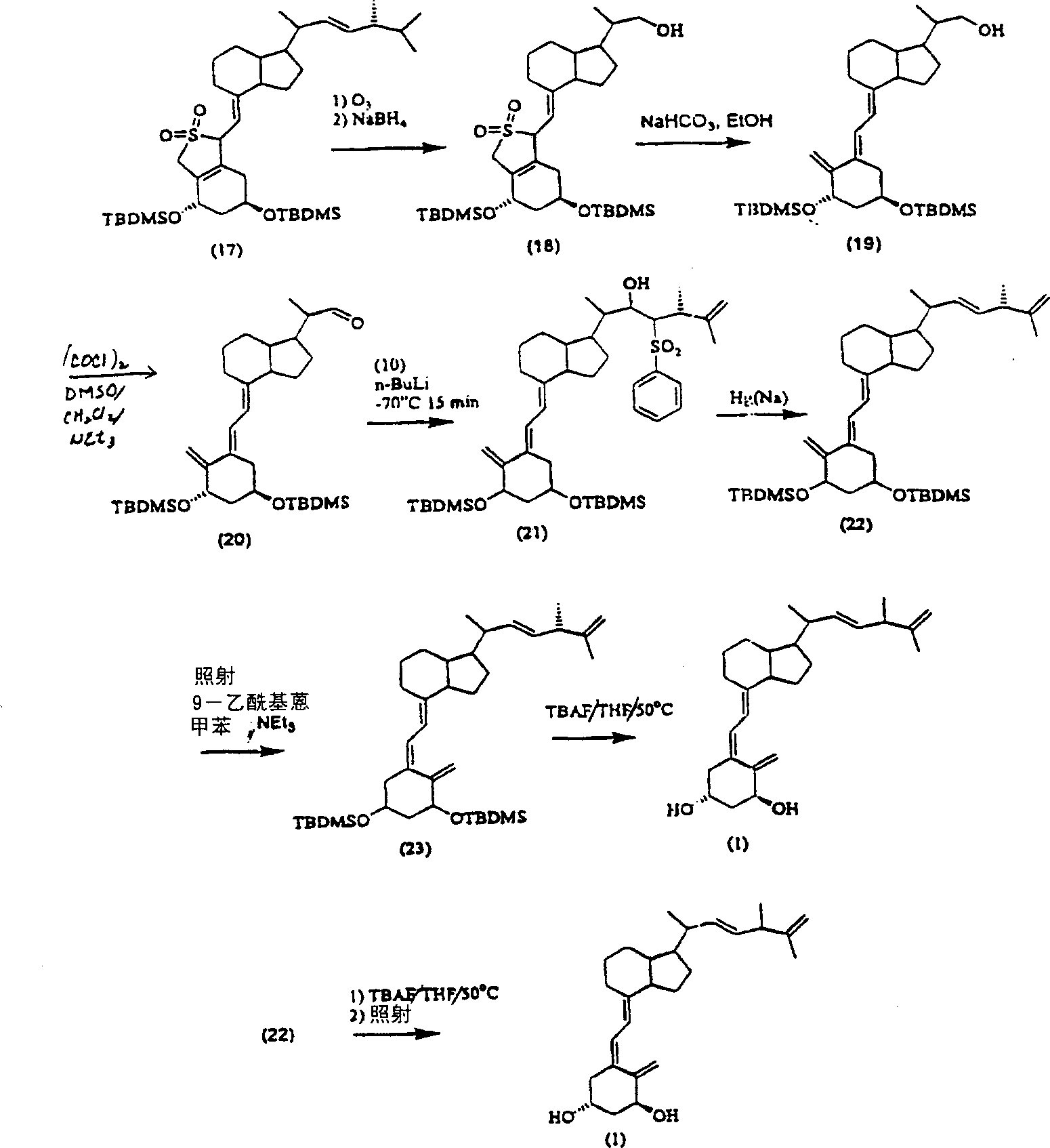
[0094] To a solution of 320 mg (22) in THF was added 400 mg TBAF. The mixture was stirred overnight at room temperature, reacted at 55 °C for 3 hr and poured into saturated NaHCO 3 in solution. The mixture was extracted with EtOAc (2x50ml), the organic phase was washed with brine, dried and the solvent was evaporated. Column chromatography (silica gel, (EtOAc-hexane 2:1)) separated to give a white solid, which was dissolved in 40ml of toluene, after adding 6 drops of NEt 3 And 5mg 9-acetyl anthracene post-irradiation for 1.5hr. Chromatography (silica gel, (EtOAc-hexane 2:1)) after evaporation of the solvent afforded 65 mg (31%) of 1α-hydroxy-25-ene-vitamin D2(1) with a purity of 96.7% (HPLC). UV:λ max 265nm. Example 2: 24-Hydroxy-25-ene-Vitamin D 2 synthesis
[0095]Except that the hydroxylation step at the C-1 position is omitted and no protecting group is added at the C-1 position, 24-hydroxy-25-ene-vitamin D 2 The synthesis of is identical with the step of embodimen...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap