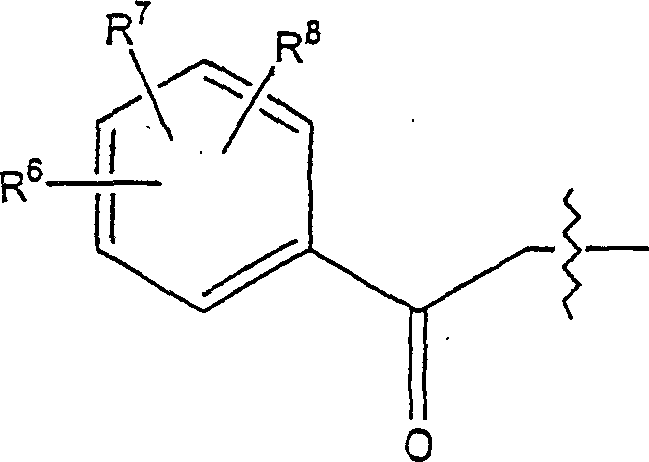
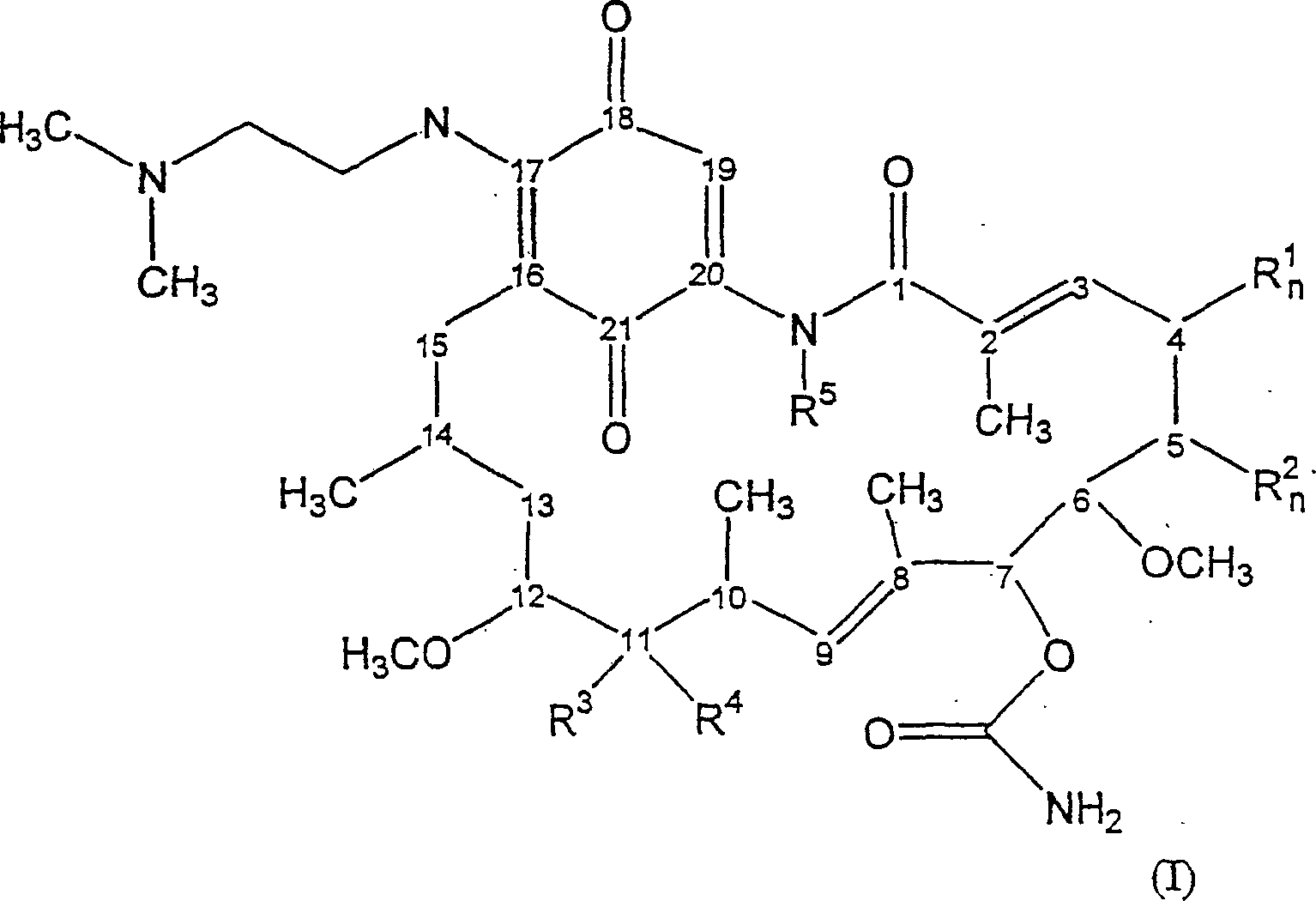
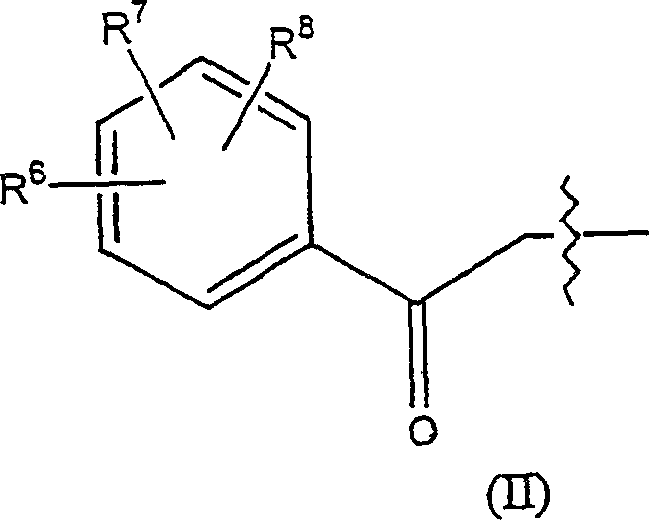
Geldanamycin derivative and method of treating cancer same
A technology of geldanamycin and derivatives, applied in the field of geldanamycin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] This example shows the in vivo activity of compounds of the invention compared to geldanamycin derivatives of similar structure.
[0067] Compounds A-C were subjected to hollow fiber assays as described above. Compounds A and B were mixed with saline and Tween 80 (0.05%) to give two dose levels of 75 mg / kg / dose and 50 mg / kg / dose, respectively. Compound C was also mixed with saline and Tween 80 (0.05%) to give two dose levels of 20 mg / kg / dose and 13.4 mg / kg / dose, respectively. Optimal dosage levels were determined from previous toxicology studies, therefore, the dosage used represents the upper limit that can be administered to the host without inducing toxic effects. All three compounds were administered by intraperitoneal injection once daily for four days. After 4 days, the hollow fibers were removed from the mice and the MTT assay described above was performed. The HFA score for each compound was determined from the calculation of percent cell growth inhibition [(...
Embodiment 2
[0071] This example shows the oral in vivo activity of the compounds of the invention.
[0072]As described above, hollow fiber detection was performed using compound B of the present invention. Compound B was mixed with water to give two dosage levels of 75 mg / kg / dose and 50 mg / kg / dose, respectively. Compound B was then administered orally (PO) once a day for four days. After 4 days, the hollow fibers were removed from the mice and the MTT assay described above was performed. The HFA score for each compound was determined from the calculation of percent cell growth inhibition [(1-(% treated net growth / % control net growth))*100], as shown in Table 2.
[0073] compound
[0074] According to the above results, Compound B of the present invention exhibited significant in vivo anticancer activity in oral administration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com