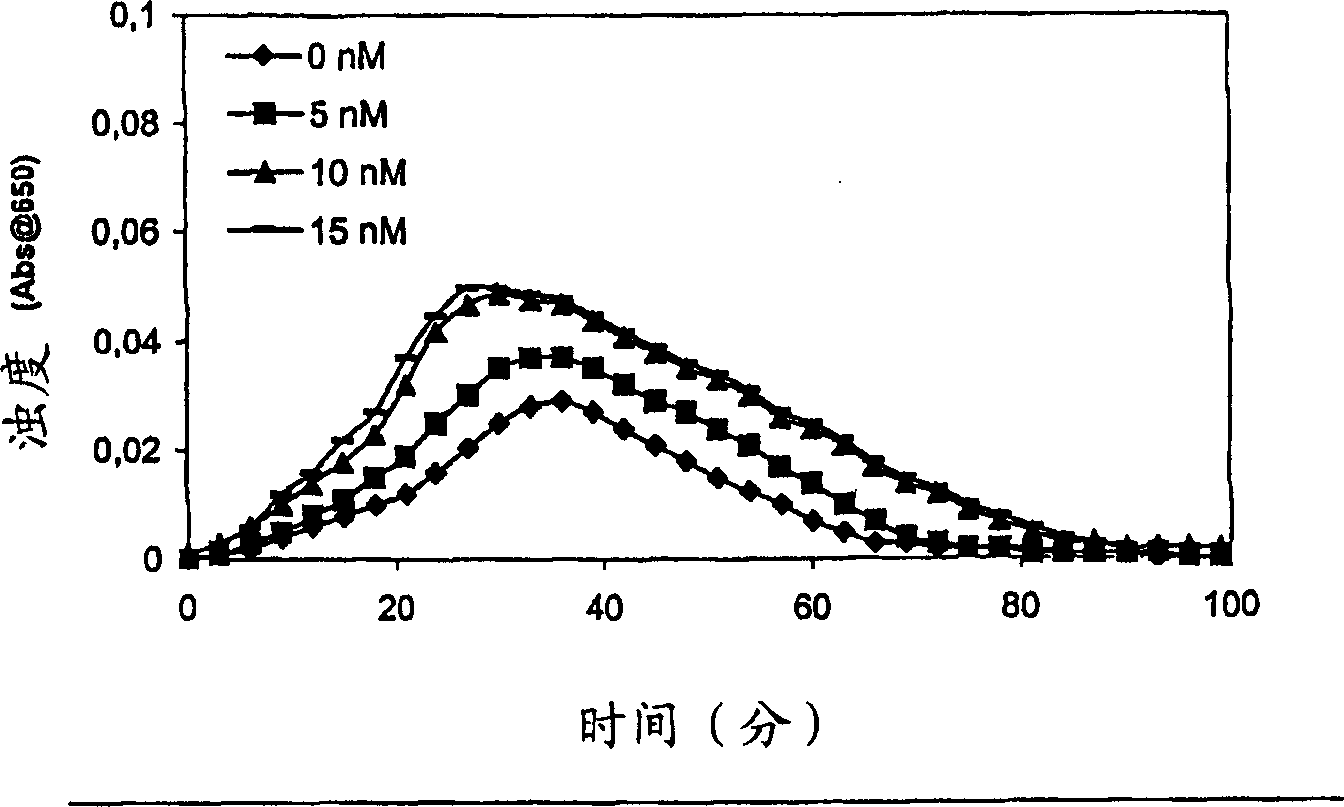
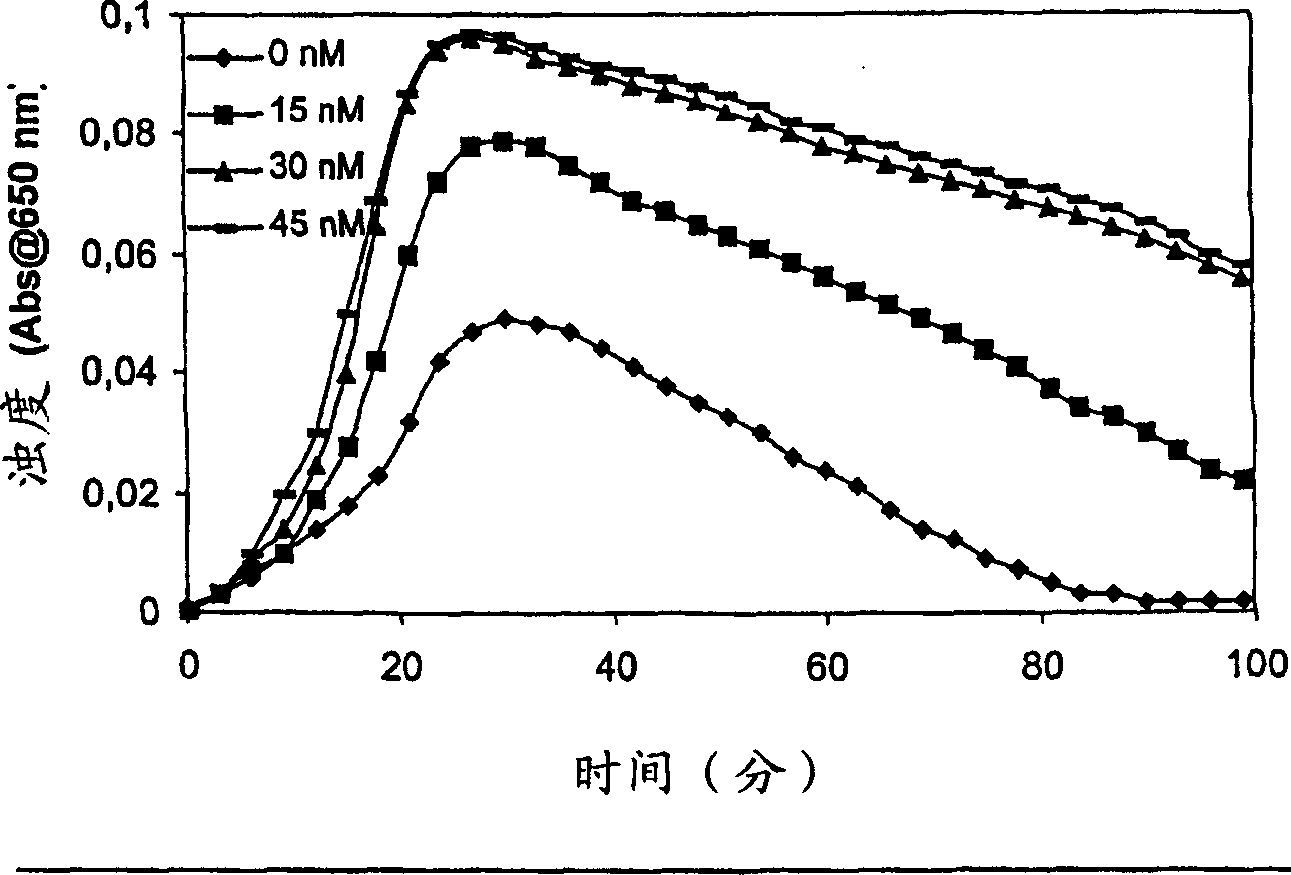
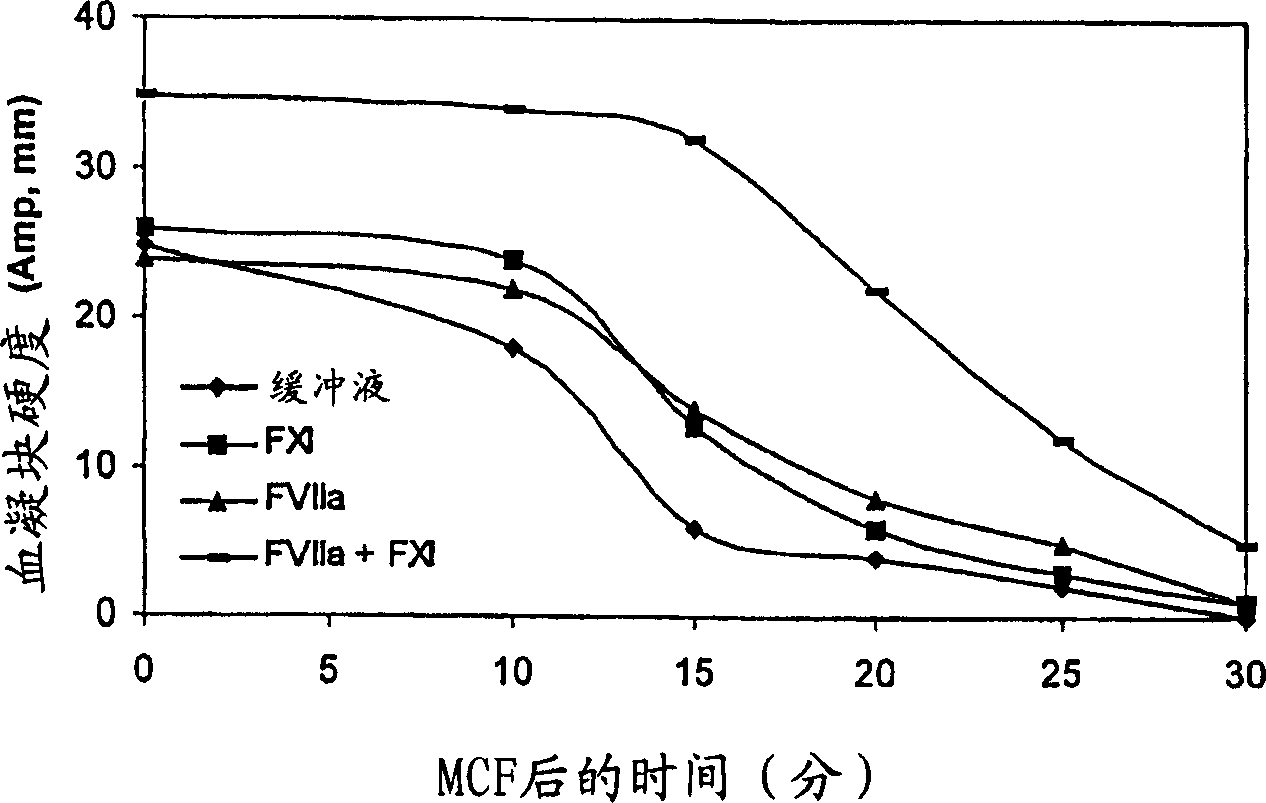
Pharmaceutical composition comprising factor VII polypeptides and factor XI polypeptides
A technology of composition and factors, applied in the preparation and treatment of subjects suffering from bleeding episodes, drugs for treating subjects with bleeding episodes, preventing their episodes, and promoting the formation of blood coagulation in subjects, which can solve difficult and unsuccessful treatments And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0186] Compound preparation:
[0187] The human purified factor VIIa suitable for use in the present invention is preferably obtained by DNA recombinant technology, for example according to Hagen et al., Proc. Natl. Acad. Sci. USA 83 : 2412-2416, 1986, or prepared as described in European Patent No. 200,421 (ZymoGenetics, Inc.).
[0188] Factor VII is also available according to Broze and Majerus, J.Biol.Chem.255 (4): 1242-1247, 1980 and Hedner and Kisiel, J. Clin. Invest. 71 : 1836-1841, produced by the method described in 1983. These methods yield Factor VII without detectable amounts of other coagulation factors. Even more purified Factor VII preparations can be obtained by including an additional gel filtration as a final purification step. Factor VII can then be converted to activated factor FVIIa by known methods, for example by several different plasma proteins, eg Factor XIIa, IX or Xa. In addition, according to Bjoern et al. (Research Disclosure, 269 , Septem...
Embodiment approach 2
[0242] Embodiment 2: The composition of aspect 1, wherein said Factor VII or Factor VII-related polypeptide is a Factor VII-related polypeptide.
Embodiment approach 3
[0243] Embodiment 3: The composition of Aspect 1, wherein Factor Vila is human Factor Vila.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com