Reversal binding agents for anti-factor XI/XIA antibodies and uses thereof
A binding agent and antibody technology, applied in the direction of anti-coagulation factor immunoglobulin, anti-animal/human immunoglobulin, antibody, etc., can solve problems such as unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0510] Human Fab Phage Library Panning
[0511] Using commercially available phage display library Morphosys HuCAL The library was used as a source of antibodies, and antibodies against NOV1401 were generated by selecting clones that combined with NOV1401. Phagemid libraries are based on Concept ((Knappik et al., 2000, J Mol Biol 296:57-86), and use CysDisplayTM technology to display Fab on the surface of phage (WO01 / 05950). In order to isolate anti-NOV1401 antibodies, a solid-phase panning strategy was used to directly extract NOV1401 Package was delivered to Maxisorp TM (Nunc) 96-well plate, followed by three rounds of panning with gradually increasing wash stringency.
[0512] Subcloning and microexpression of selected Fab fragments
[0513] To facilitate the rapid expression of soluble Fab, the selected HuCAL The Fab-encoding insert of the phage was derived from The display vector was subcloned into Expression vector middle.
[0514] For initial screening an...
Embodiment 2
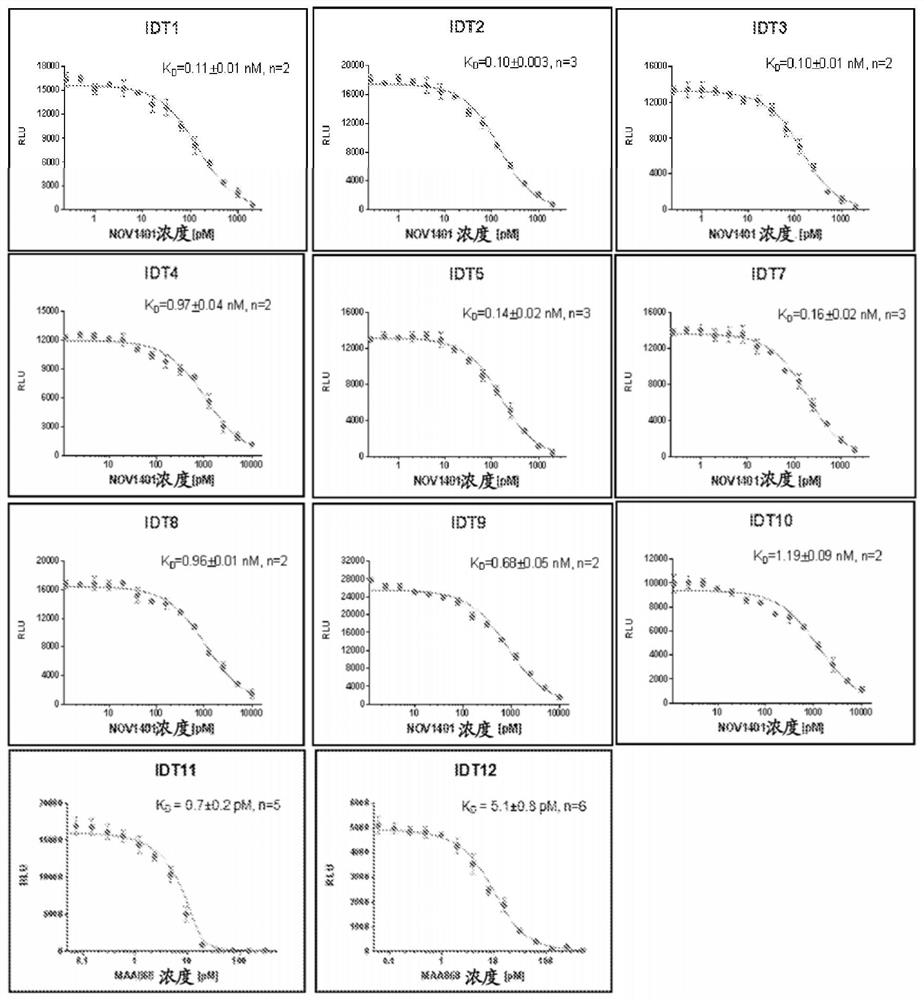
[0528] combine data
[0529] Surface Plasmon Resonance (SPR) Binding Analysis of Anti-NOV1401 Fab Binding to NOV1401
[0530] SPR binding experiments were performed on a ProteOn XPR36 instrument (Bio-Rad Laboratories, Inc.) at 25°C in PBS / T buffer (50 mM phosphate, 150 mM NaCl, pH 7.4, 0.005% v / v Tween-20). NOV1401 ("ligand") was immobilized on an activated ProteOn GLC sensor chip (Bio-Rad Laboratories, Inc.) using standard amine coupling procedures as described by the manufacturer. Briefly, NOV1401 at a concentration of 10 μg / ml in 20 mM sodium acetate, pH 5.0 was injected at a flow rate of 30 μl / min for 10 minutes. Unreacted groups were blocked by injection of 1M ethanolamine.
[0531] For kinetic studies, anti-NOV1401 Fab ("analyte") was diluted in PBS / T buffer to generate a dilution series with concentrations ranging from 0.125-4 nM. The Fab was injected into the NOV1401-immobilized surface at a flow rate of 100 μL / min, and the sensorgram was recorded, the association a...
Embodiment 3
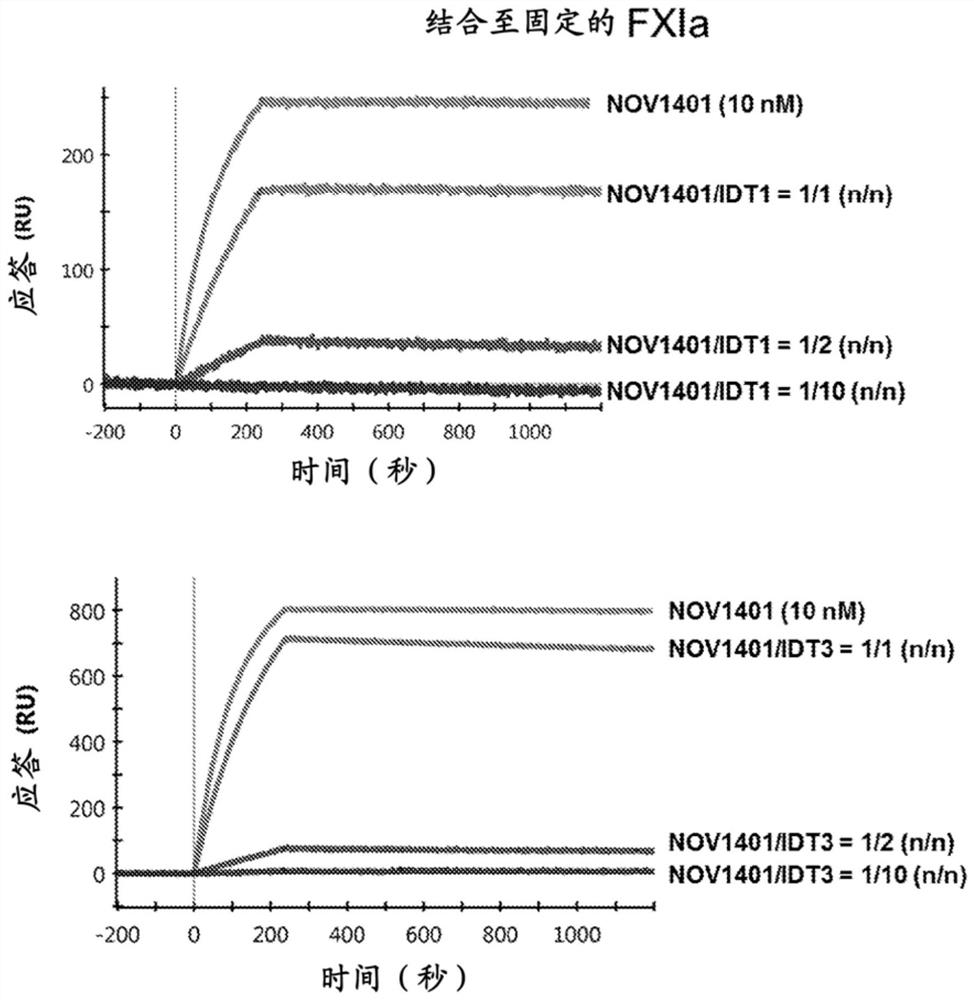
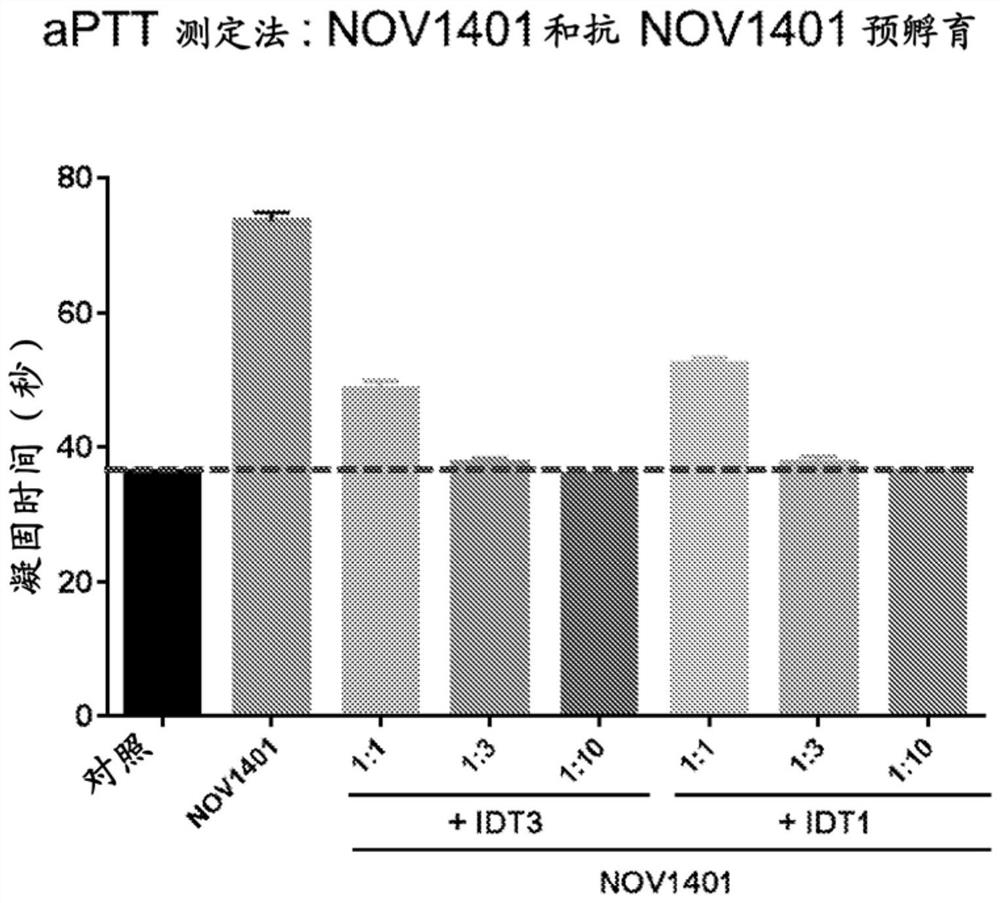
[0546] SPR binding competition
[0547] SPR experiments were performed essentially as described in Example 2 with the following changes. Human plasma-derived FXIa was used as ligand and immobilized on activated ProteOn GLC sensors by injecting FXIa at a concentration of 10 μg / ml in 20 mM sodium acetate, pH 5.0, at a flow rate of 30 μl / min for 10 min using the standard amine coupling procedure as described On a chip (Bio-Rad Laboratories, Inc.).
[0548] For binding competition studies, three mixtures of NOV1401 and anti-NOV1401 Fab at molar ratios of 1:1, 1:2, and 1:10 were prepared in PBS / T buffer and injected simultaneously at a flow rate of 100 μL / min into immobilized cells. FXI on the surface. Sensorgrams were recorded with association and dissociation times of 220 s and 1800 s, respectively. Blank surfaces are used for background correction.
[0549] The NOV1401 / anti-NOV1401 Fab mixture produced a significantly lower binding response to immobilized FXIa than NOV1401 a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com