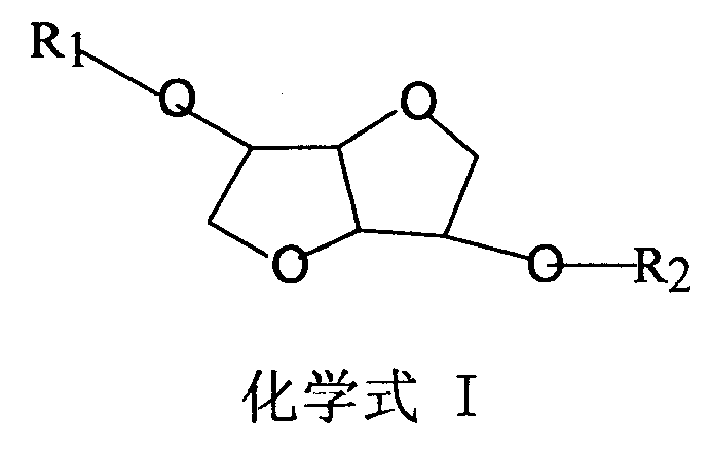
Preparation of selective epoxide hydrolase II inhibitor
A cyclooxygenase and inhibitor technology, applied in the field of new pharmaceutical preparations, can solve problems such as the inability to provide cyclooxygenase 2 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0049] While stirring, 3.976 mmol of rofecoxib was added to 100 ml of 2,5-bis-O-methyl-1,4:3,6 dianhydro-D-glucitol. The solution was stirred for an additional 15 minutes under aseptic conditions. The sterile-filtered solution is sealed in vials / ampoules.
example 2
[0051] While stirring, 3.976 mmol of rofecoxib was added to 80 ml of 2,5-bis-O-methyl-1,4:3,6 dianhydro-D-glucitol. Continue to stir the solution under aseptic conditions and add 20ml of water for injection. The solution (diluted to 100 ml with 2,5-bis-O-methyl-1,4:3,6 dianhydro-D-glucitol) was sterile filtered and sealed into vials / ampoules.
example 3
[0053] While stirring, 7.952 mmol of rofecoxib was added to 80 ml of 2,5-bis-O-methyl-1,4:3,6 dianhydro-D-glucitol. The solution was continuously stirred under aseptic conditions and 20ml of water for injection was added. The solution (diluted to 100 ml with 2,5-bis-O-methyl-1,4:3,6 dianhydro-D-glucitol) was sterile filtered and sealed into vials / ampoules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com