Redox active reversible electrode and novel cell using it
An active and electrode technology, which is applied in the field of redox active reversible electrodes, can solve the problems of low conductivity or low conductivity, difficulty in increasing the electric energy of the battery, and difficulty in selecting the best conditions for the reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Among the thiophene compounds represented by formula (II), specially formulated EDOT polymer containing 20mM and 0.1M lithium perchlorate (LiClO 4 ) in acetonitrile (AN) solution (solution for electrolytic polymerization).
[0075]PEDOT covered electrodes were fabricated as follows. A 3-electrode battery is used, using an enamel carbon disc electrode with a diameter of 3mm as the working electrode, a coil-shaped platinum wire as the counter electrode, and a silver ion electrode as the reference electrode, and passing through the above-mentioned solution for electrolytic polymerization. Electrolytic oxidative polymerization was performed to fabricate PEDOT-covered electrodes. 0.05M silver perchlorate was dissolved in the used solvent (AN), and this was used as an internal solution, and a silver ion electrode was fabricated using a commercially available electrode holder. In addition, the enamel carbon disk electrode was used after polishing with alumina for polishing o...
Embodiment 2
[0078] As a representative example of organic sulfur-containing compounds, 2,5-dimercapto-1,3,4-thiadiazole (DMcT) was selected, and 1.0 M LiClO containing 5 mM DMcT was prepared. 4 AN solution, or 1.0M LiClO 4 1.0M LiBF dissolved in a solution of N-methyl-2-pyrrolidone (NMP) or a mixture of propylene carbonate (PC) and ethylene carbonate (EC) at a weight ratio of 1:1 4 of electrolyte. The CV measurement was carried out by using the battery in which the crushed carbon electrode as the working electrode was immersed in the above-mentioned electrolyte solution and the PEDOT film covered the crushed carbon electrode prepared by the method in Example 1.
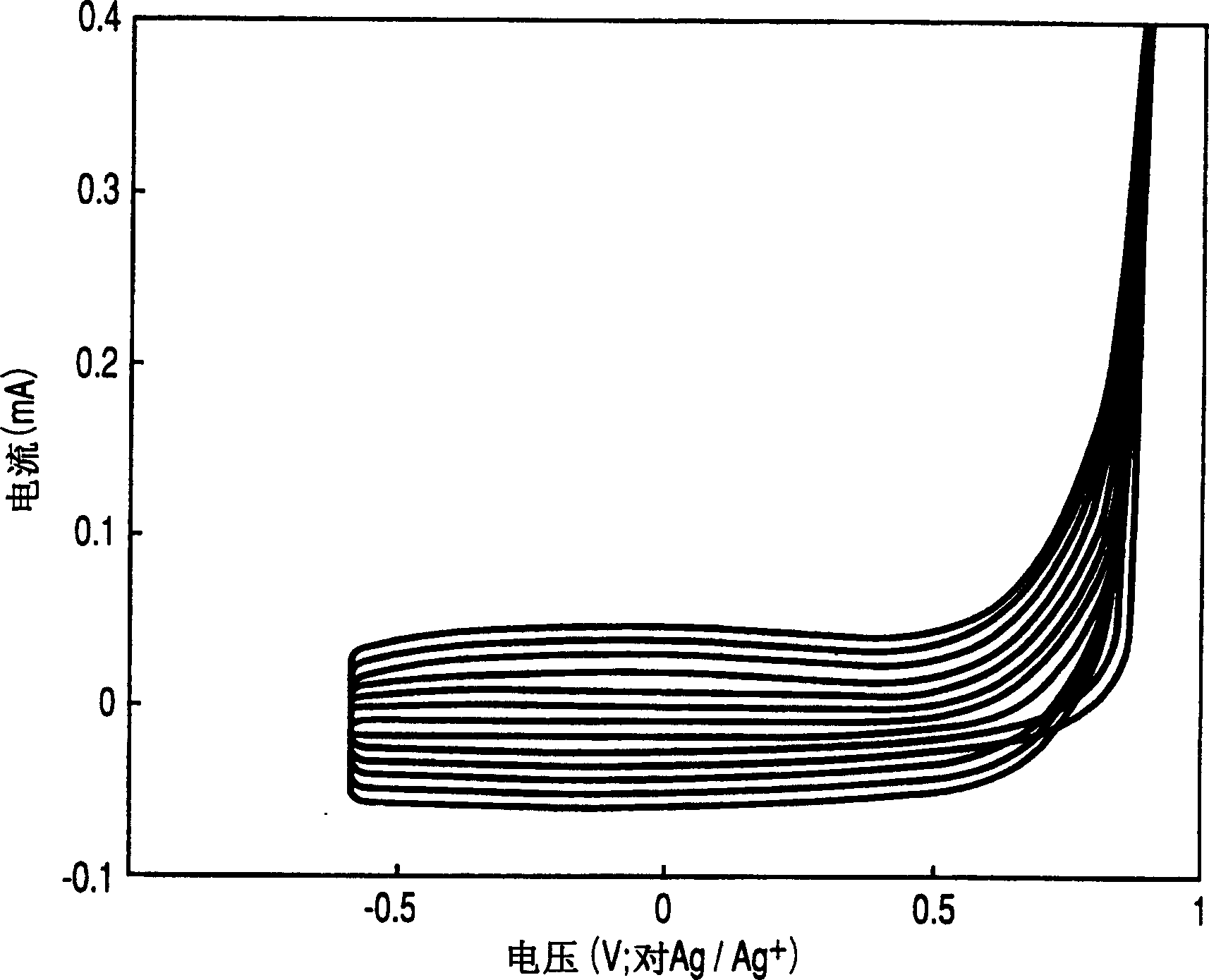
[0079] Figure 4A and Figure 4B , is the CV of DMcT measured in AN electrolyte with the electrode not covered with PEDOT film and the electrode covered with PEDOT film, respectively. The measurement is carried out by changing the potential scanning range. exist Figure 4A , Figure 4B The CVs after potential sweeping in t...
Embodiment 3
[0083] by containing 0.1M LiClO 4 A measurement solution was prepared by adding DMcT to 2 mM NMP. As a working electrode, an enamel carbon disc electrode (3 mm in diameter) for a rotating electrode was used. By the same operation as in Example 2, a PEDOT-covered electrode was produced. The measurement was performed using a coiled platinum wire as a counter electrode and a silver ion electrode as a reference electrode. exist Figure 5 In , the current-potential curve corresponding to the oxidation reaction of DMcT from monomer to dimer in the PEDOT film-covered electrode and the uncovered electrode obtained at a rotational speed of 400 (rotation / minute) is shown. Figure 5 Curve (a) in , is the current-potential curve obtained with uncovered electrodes. It is observed that the limiting current increases with the increase of rotational speed. In addition, the half-wave potential is shifted towards the positive pole as the rotational speed increases. Figure 5 The curve (b)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com