Method for desulphurisation of natural gas
A removal, gaseous technology, applied in the direction of separation methods, chemical instruments and methods, gas fuels, etc., can solve the problem of high commercial costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
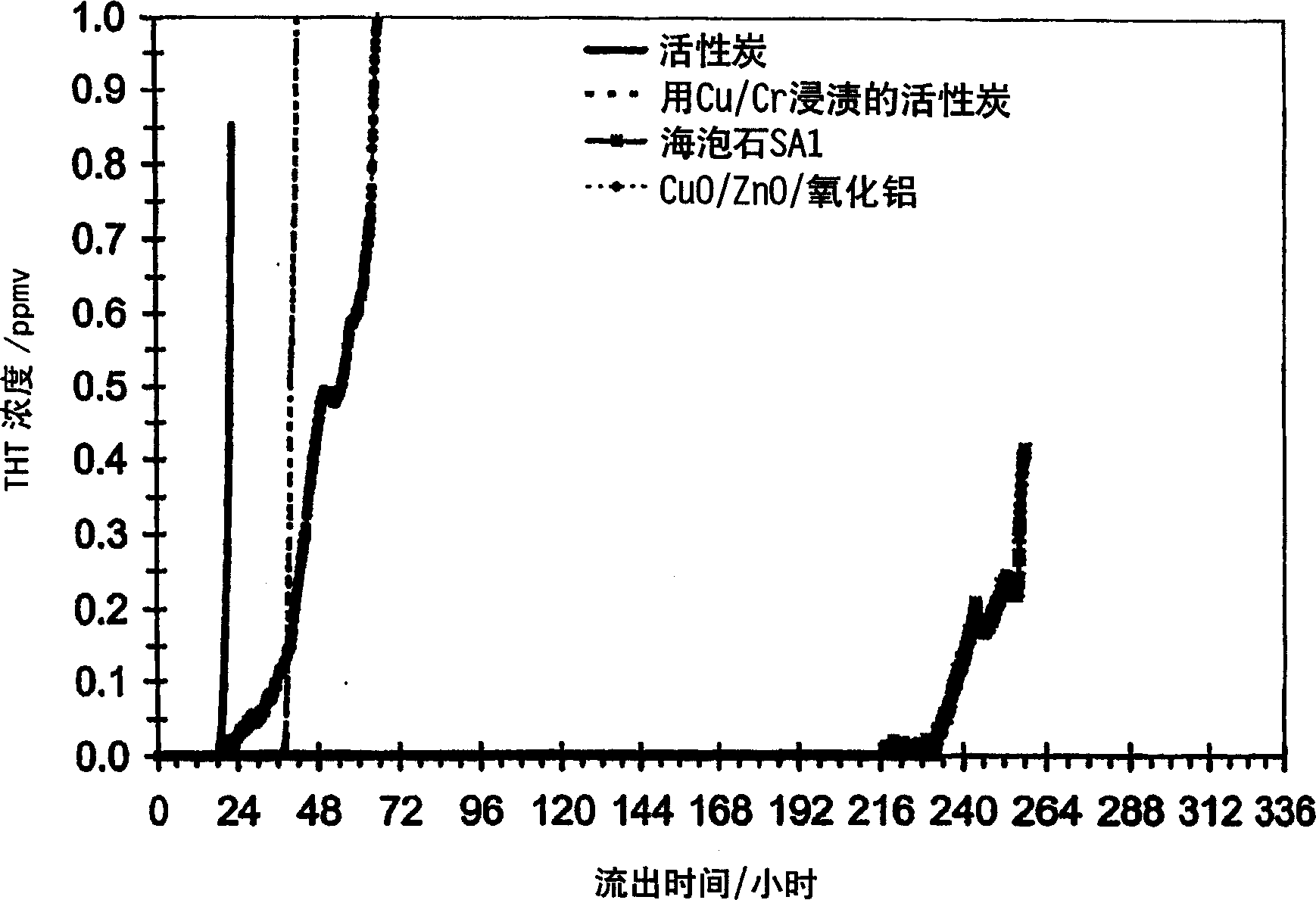
[0075] In this example sepiolite (obtained as dust-free catlitter granules, >80% (m / m) sepiolite and <20% (m / m) zeolite) With many commonly used adsorbents such as activated carbon (Norit, code RB1; peat-based, steam-activated, extruded, unimpregnated), activated carbon impregnated with copper and chromium (Norit, code RGM1; peat-based, steam-activated and impregnated ); and copper oxide / zinc oxide / alumina (BASFR3-12; metal / metal oxide) for comparison. The sepiolite of the present invention is capable of binding the most sulfur.
[0076]
[0077] THT adsorption test results
[0078] The THT efflux curve of the above-mentioned adsorbent samples (the concentration of THT in natural gas after filtering is plotted against the efflux time) at figure 1 given in. The efflux curves clearly show that sepiolite (sepiolite sample SA1) adsorbs 5 to 10 times more THT than activated carbon and copper oxide / zinc oxide / alumina materials. With the exception of sepiolite, a highly...
Embodiment 2
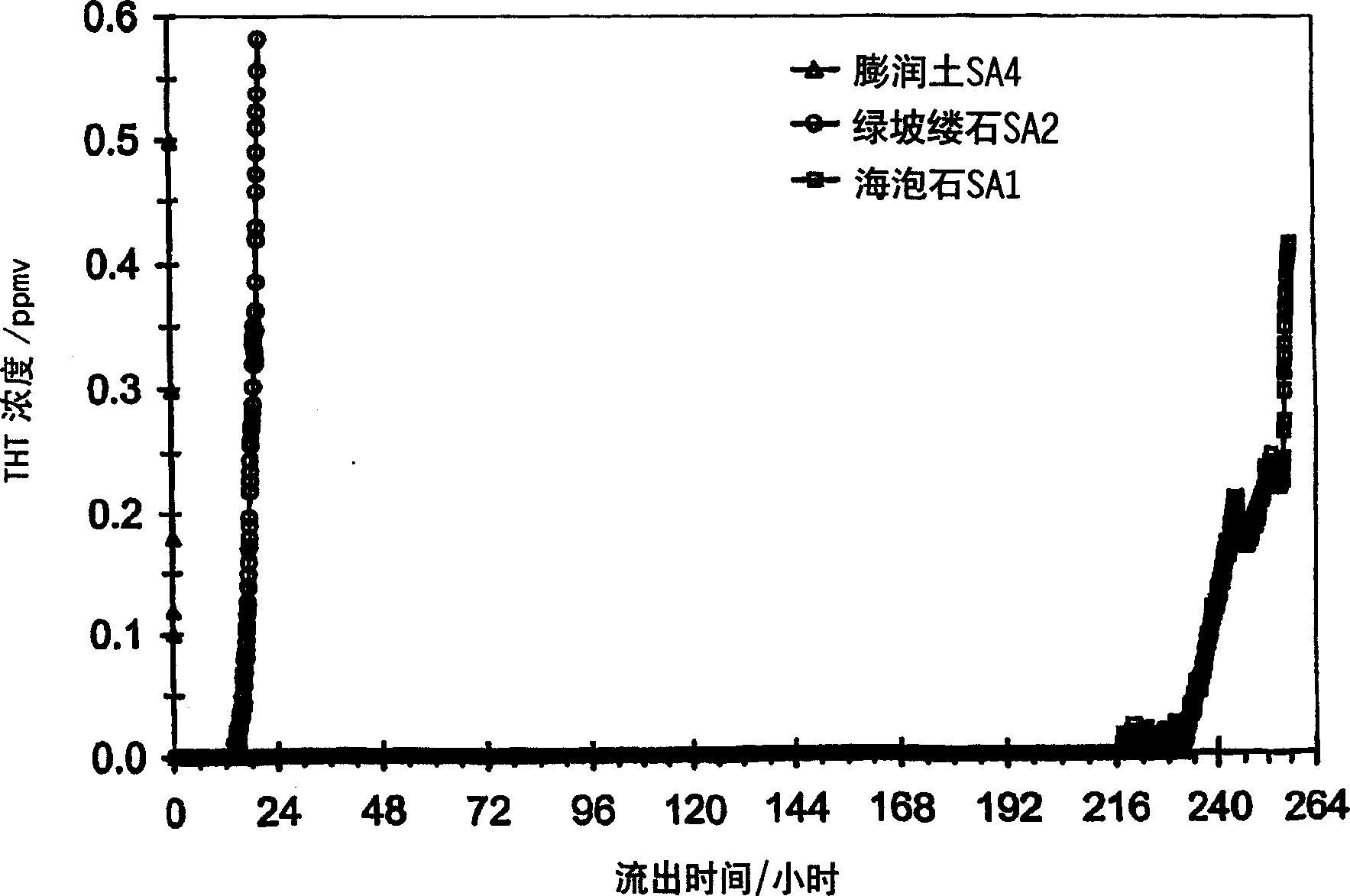
[0083] In this example, sepiolite (the same as in Example 1) was mixed with attapulgite (calcined clay particles, 85% (m / m)) under the same conditions as in Example 1 as the refractory soil waste. Attapulgite, from Tijssen, Hazerswoude, The Netherlands) and a naturally occurring clay mineral, bentonite (refractory clay granules, agglomerates, coarse material). These materials can be obtained from grocery stores and the like.
[0084] refer to figure 2 , it can be seen that compared with these materials, sepiolite (sample SA1) is able to adsorb more sulfur than bentonite (sample SA4) and attapulgite (sample SA2).
[0085] Experimental equipment and test conditions of embodiment 3-6
[0086]The present adsorption experiments were carried out in a manually operated flow plant connected to a natural gas supply network at 100 mbar (o) (o = overpressure) by means of two on / off taps and an overflow protection device (needle valve) middle. (Cylinder) gas can also be connected via...
Embodiment 3-5
[0096] Examples 3-5: Impregnation with copper
[0097] Accurately weigh 25 grams of sepiolite with a particle size of 0.5-1.0 mm and place in a glass beaker. In the so-called "dry impregnation" (incipient wetness), the amount of sepiolite is capable of absorbing a maximum of about 40 ml of water. Then, 1.57 grams of copper acetate was weighed out and dissolved in approximately 40 milliliters of demineralized water in a beaker with the aid of approximately 10 minutes of room temperature vibration in an ultrasonic bath. Then, the sepiolite was impregnated with the resulting solution by dry impregnation. After brief manual stirring, the impregnated sepiolite was dried in a drying cabinet at 40° C. for a minimum of 24 hours in air. Material dried in this way contained approximately 2% (m / m) Cu 2+ , and are ready for adsorption assays. Prepared in the same manner as above containing 5% (m / m) Cu 2+ sample.
[0098] Results: Table 4 shows the measured adsorpt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap