Medication in use for releasing fatigue and preparation method
A technology for relieving fatigue and drugs, applied in drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Mint 575, Borneol 340, Chuanxiong 325, Woody Fragrance 540, Licorice 450, Gouteng 360, Tribulus terrestris 450, Ziziphus 450
[0033] The crude drug of above-mentioned weight part is prepared according to the following method:
[0034] ① Pulverize the raw materials except the woody incense and borneol and pass through the No. 2 sieve; crush the woody woody powder with a water content lower than 1% and sieve, and mix the obtained fine powder evenly;
[0035] ②Put the borneol in white wine at a temperature of 5°C until it is completely dissolved, wherein the weight of the white wine is 0.5 times the weight of the borneol;
[0036] ③ The fine powder obtained in step ① and the solution obtained in step ② are mixed evenly and stirred, and placed in an airtight container at a temperature of 20°C for 140 hours;
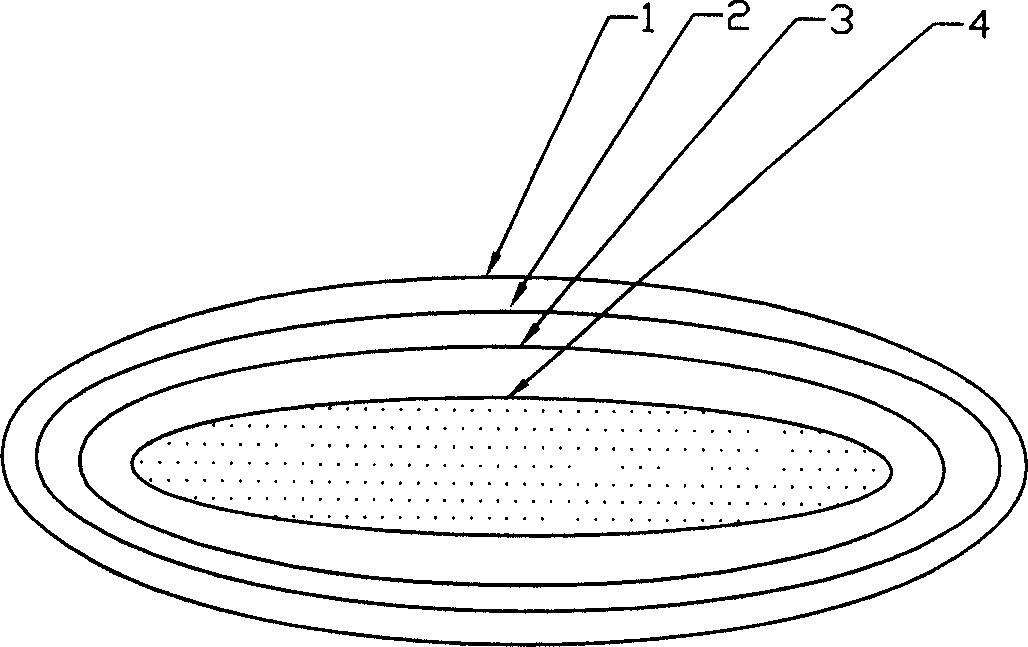
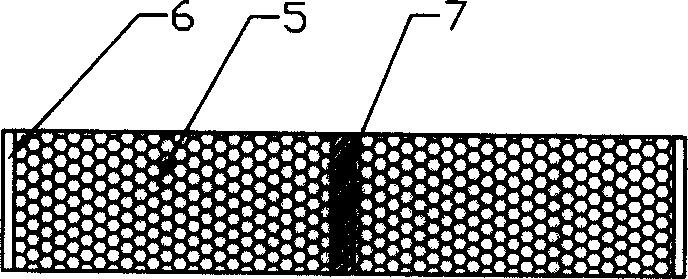
[0037] ④ in figure 1 168 holes 5 are respectively drilled around the boundary line 7 of the plane single layer of the shown plastic envelope; step 3. the obtained m...
Embodiment 2
[0039] Mint 640, Borneol 380, Chuanxiong 360, Woody Fragrance 600, Licorice 500, Gouteng 400, Tribulus terrestris 500, Ziziphus 500
[0040] The crude drug of above-mentioned weight part is prepared according to the method for embodiment 1:
[0041] ① Pulverize the raw materials except the woody incense and borneol and pass through the No. 2 sieve; crush the woody woody powder with a water content lower than 1% and sieve, and mix the obtained fine powder evenly;
[0042] ②Put the borneol in white wine at a temperature of 10°C until it is completely dissolved, wherein the weight of the white wine is twice the weight of the borneol;
[0043] ③ The fine powder obtained in step ① and the solution obtained in step ② are mixed evenly and stirred, and placed in a closed container at a temperature of 25°C for 130 hours;
[0044] ④ in figure 1 170 holes 5 are respectively drilled around the boundary line 7 of the plane single layer of the shown plastic envelope; step 3. the obtained ...
Embodiment 3
[0047] Mint 600, Borneolum 350, Chuanxiong 365, Woody Fragrance 580, Licorice 480, Gouteng 400, Tribulus terrestris 475, Ziziphus 500
[0048] The crude drug of above-mentioned weight part is prepared according to the following method:
[0049] ① Pulverize the raw materials except the woody incense and borneol and pass through the No. 2 sieve; crush the woody woody powder with a water content lower than 1% and sieve, and mix the obtained fine powder evenly;
[0050] ②Put the borneol in alcohol at a temperature of 15°C until it is completely dissolved, wherein the weight of the alcohol is 1 times the weight of the borneol;
[0051] ③ The fine powder obtained in step ① and the solution obtained in step ② are mixed evenly and stirred, and placed in a closed container at a temperature of 30°C for 80 hours;
[0052] ④ in figure 1 180 holes 5 are respectively drilled around the boundary line 7 of the plane single layer of the shown plastic envelope; step 3. the obtained material i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com