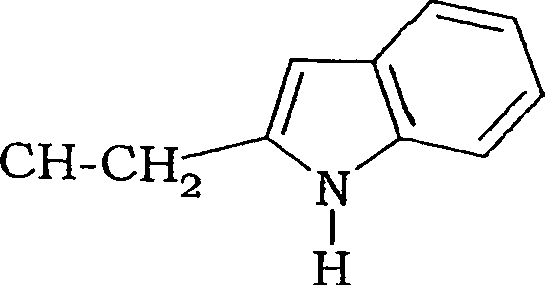
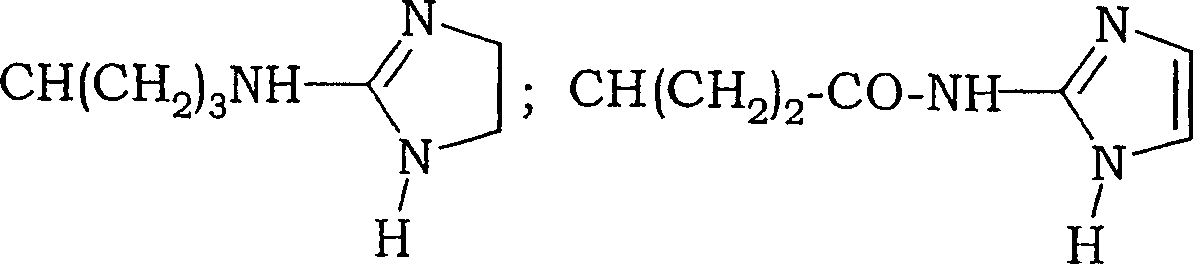
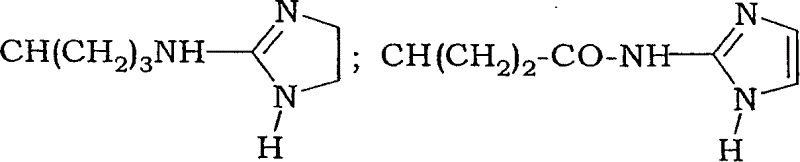Fluoro-alkyl-cyclopeptide derivatives having anti-integrin activity
A compound and drug technology, applied in the field of fluoro-alkyl-cyclic peptide derivatives, can solve problems affecting proteolytic stability and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] C(Arg-Glv-Asp-D-Phe-(R or S)-Tfm-Phe) (ST1930 / ST1931)
[0080] Preparation of Pht-D-Phe-Br (Dal Pozzo, A., Bergonzi, R. and Ni, M.H., Tetrahedron Lett., 2001, 42, 3925-7).
[0081] 1.4 g (4.75 mmol) Pht-D-Phe-OH were dissolved in 19 ml of a 0.5 M solution of bromoenamine in DCM under argon. After 10 minutes, the solution is ready to use.
[0082] Add 248mg (0.950mmol) of H-α-Tfm-Phe-OEt and collidine (1 equivalent) to 12.5ml of bromide solution (prepared as above) cooled to 0°C, stir the mixture at room temperature (a.t.), after 10 minutes Another 6.5 ml portion of the bromide solution and 1 equivalent of collidine were added. After 2 h the mixture was dried with 15 ml of 5% NaHCO 3 and 15 ml EtOAc, and stirred for 30 minutes. The solvent was washed with water, 1N HCl and water, then evaporated and the residue was purified on a flash column using 8:2 hexane-EtOAc as solvent.
[0083] 4.3ml of 1M BBr 3 The DCM solution of 460 mg of dipeptide Pht-D-Phe-α-Tfm-Phe-O...
Embodiment 2
[0110] c(Arg-Gly-Asp-D-Phe-(R,S)-Dfm-Phe) (ST1932)
[0111] Proceed as described in Example 1 starting from H-(R,S)-Dfm-Phe-OEt. A mixture of the two diastereoisomers was obtained which had not yet been resolved.
[0112] 1 H-NMR (CD 3 OD): δ8.00-7.05 (d, NH), 7.35-7.15 (m, aromatics), 6.42-5.6 (t, CHF 2 ), 4.74-4.32 (m, α-CH), 4.32-3.96 (2 dd, CH 2 -Gly), 3.68-2.50(m), 1.75-1.45(m, CH 2 -CH 2 -Arg).
[0113] 19 F-NMR (CD 3 OD): δ -54.4 to -55.5.
[0114] MS (M+H + ): 673.14.
Embodiment 3
[0116] c(Arg-Gly-(R or S)-Tfm-Asp-D-Phe-Val)(ST2189 / 2190)
[0117] H-(R,S)-Tfm-Allgly-Oet was condensed with Pth-Gly-Br and then worked up as described in Example 1. With preparative HPLC, containing 22% CH 3 CN in water + 0.1% TFA resolved and purified the mixture of two diastereoisomers.
[0118] Diastereomer I (Rt 20.76)
[0119] 1 H-NMR (DMSOD 6 +D 2 O): δ7.30-7.12, 4.70, 4.26, 4.10, 3.86, 3.60-2.70, 1.72, 1.52-1.12, 0.65.
[0120] MS (M+H + ): 643.2.
[0121] Diastereomer II (Rt 25.44)
[0122] 1 H-NMR (DMSOD 6 +D 2 O): δ7.30-6.96, 4.50, 4.15, 4.10-2.75, 1.90, 1.62, 1.43-1.20, 0.82.
[0123] MS (M+H + ): 643.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com