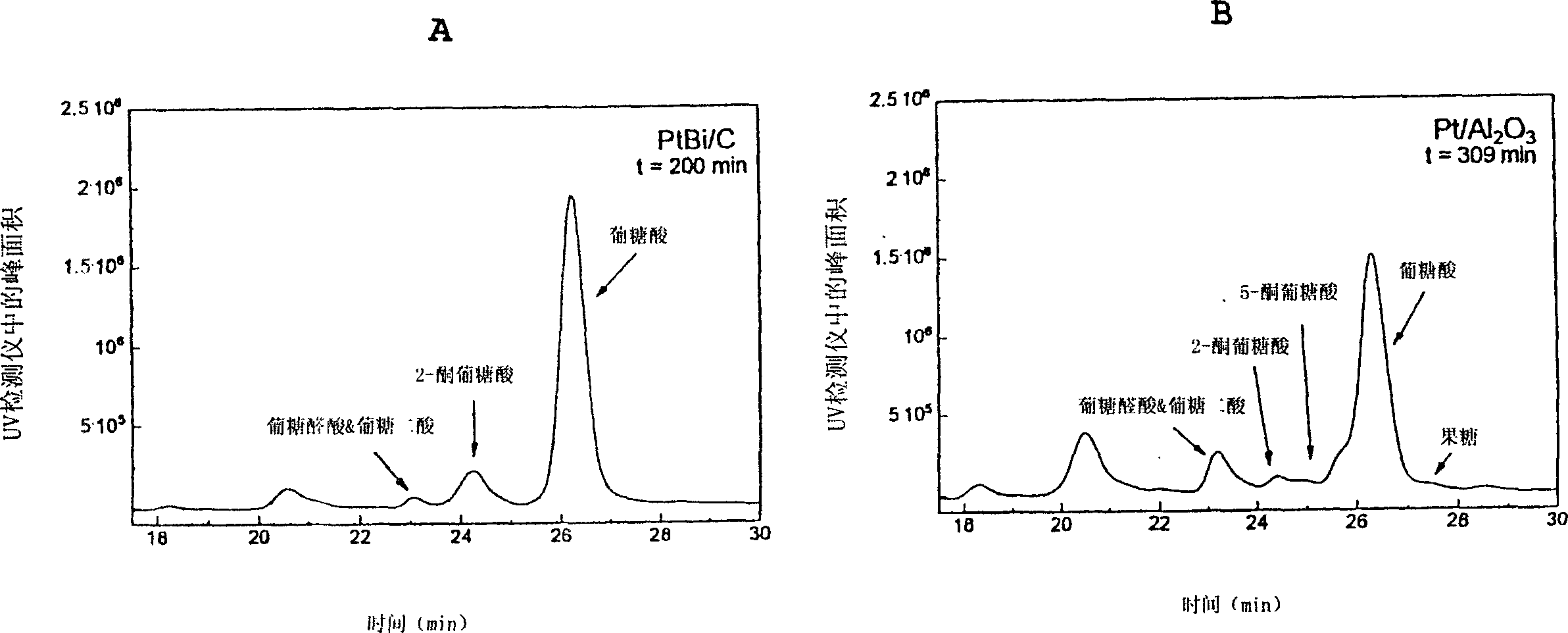
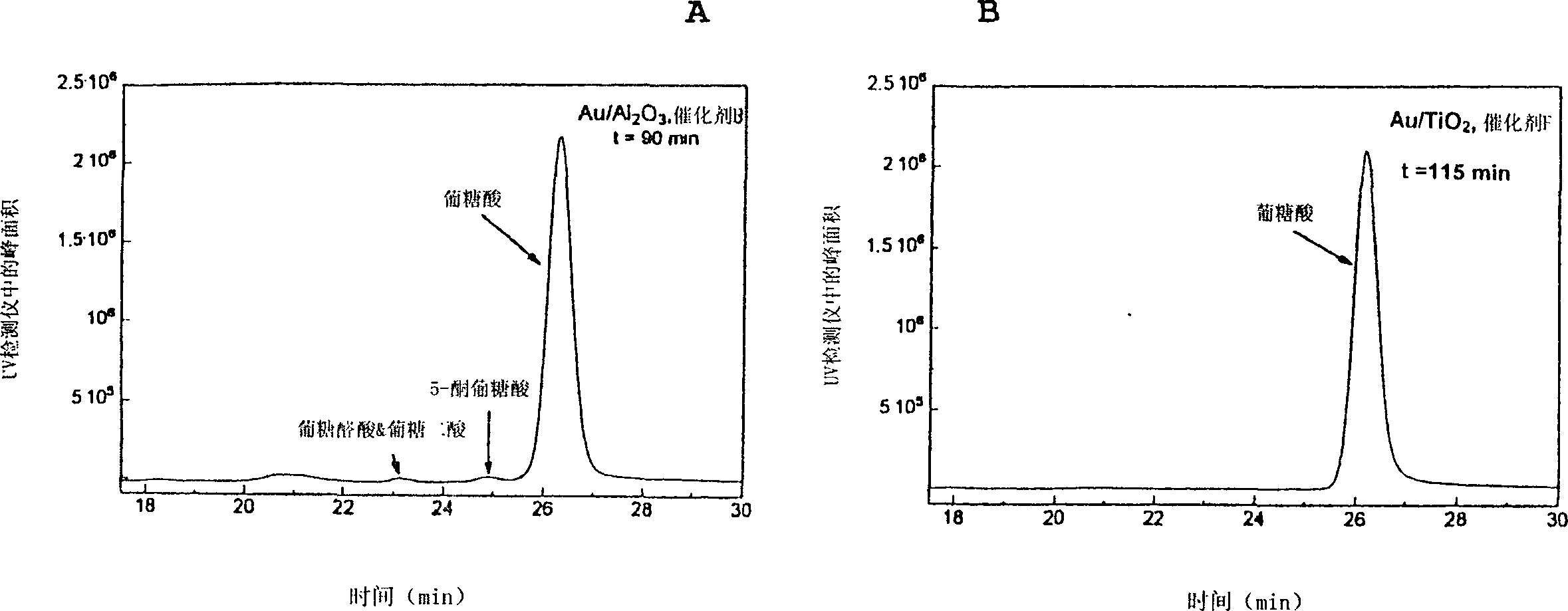
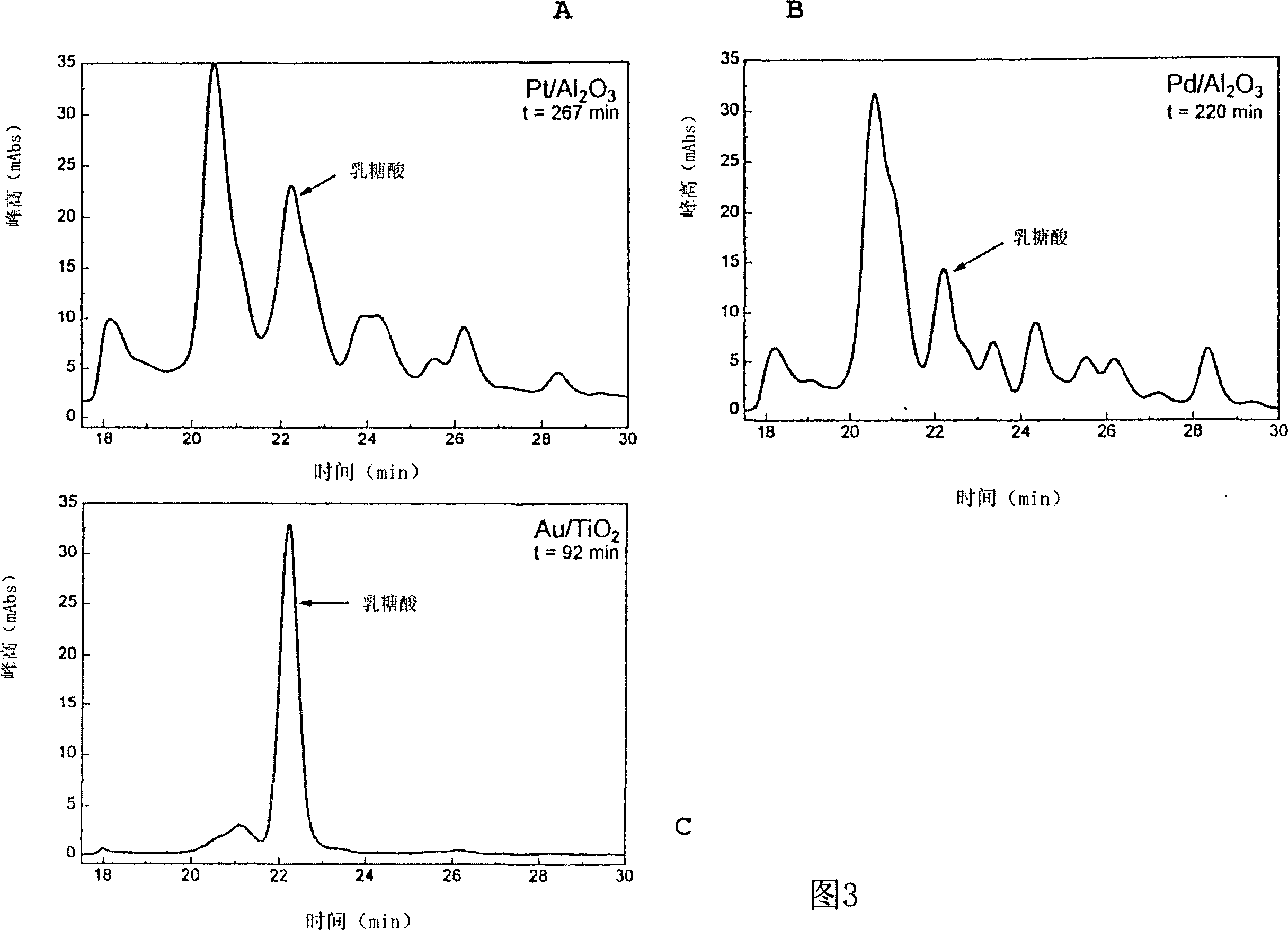
Method for selective carbohydrate oxidation using supported gold catalysts
A carbohydrate and gold catalyst technology, applied in the preparation of organic compounds, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of increased leaching, decreased catalyst activity, and promoted the growth of gold particles And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Oxidation of glucose with a gold catalyst
[0108] The following types of catalysts were used in this example:
[0109] A: 1% Au / C138 type (ACA)
[0110] B: 0.95% Au / Al 2 o 3 (ACA)
[0111] C: 0.7% Au / C11 type (ACA)
[0112] D: 1% Au / TiO 2 Type 5 (ACA)
[0113] E: 0.5% Au / TiO 2 Type 102 (ACA)
[0114] F: 0.5% Au / TiO 2 Type 149 (ACA)
[0115] Oxidation of glucose occurs under the following reaction conditions:
[0116] Reaction volume (per batch): 500mL
[0117] Catalyst dosage: 1g / L
[0118] Initial substrate concentration: 100mmol / L
[0119] pH: 11
[0120] Temperature: 40°C
[0121] Pressure: 1bar
[0122] o 2 Air flow: 500mL / min
[0123] Stirring speed: 700rpm
[0124] Table 2 shows the results obtained for the oxidation of glucose with the above-mentioned catalysts.
[0125] Table 2
[0126] nature
A
B
C
D
E
F
Glucose conversion rate (%) *
93
97
88
74
95
...
Embodiment 2
[0134] with Au / TiO 2 Catalyst to oxidize glucose
[0135] The TiO containing 0.5% Au prepared by embodiment 1 2 The supported catalyst (Catalyst G) was chosen for further studies in which the oxidation of glucose was tested under various reaction conditions.
[0136] Unless otherwise specified, the reaction conditions were the same as in Example 1.
[0137] Effect of pH on Oxidized Glucose
[0138] Using 0.5% Au / TiO 2 Table 3 shows the results of the catalyst (catalyst G) oxidizing glucose under different pH conditions.
[0139] table 3
[0140] nature
pH7
pH9
pH11
Glucose conversion rate (%) *
80
100
100
Initial activity (mmol 葡萄糖 / g 金属 min)
194
416
550
Selectivity to gluconic acid (%)
>99
>99.5
98
Selectivity to other oxidation products (%) **
<0.5
0
<0.5
Selectivity to fructose (%)
0
0
1
[0141] * Conversion rate at t...
Embodiment 3
[0159] In oxidized glucose, Au / TiO 2 Catalyst long-term stability
[0160] Tested by trial and error with 0.5% Au / TiO 2 Long-term stability of the catalyst (catalyst G in Example 1). For this purpose, the oxidation of glucose is carried out throughout the day. In the evening or over the weekend, the catalyst is placed in a reaction solution consisting mainly of an aqueous solution of gluconic acid so that the catalyst can be deposited. On the second day, the supernatant was decanted gently, and the reactor was filled with freshly prepared glucose solution, and then the second oxidation of glucose was carried out. A total of 17 batches of experiments were repeated in this way, and the reaction conditions were pH=9, temperature 40° C., initial concentration of glucose 250 mmol / L (equivalent to about 4.5% glucose / L). Further reaction conditions are as in Example 1. The results of the tests for some selected batches are listed in Table 6.
[0161] Table 6
[0162] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com