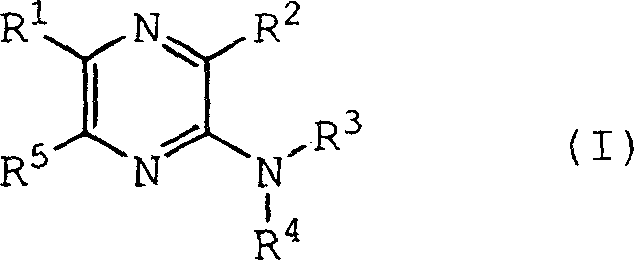
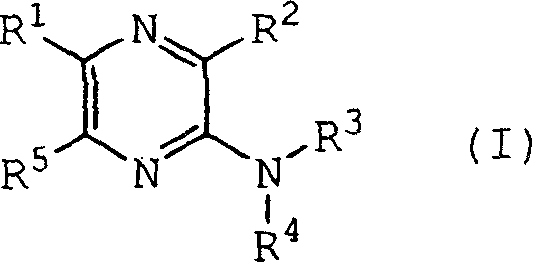
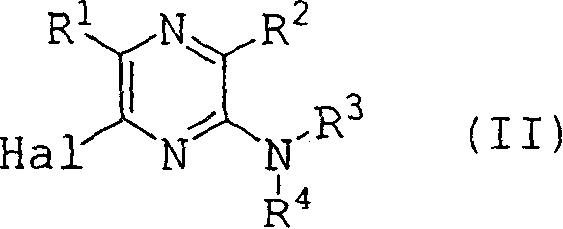
Pyrazine derivatives and pharmaceutical use thereof
A compound and low-level technology, applied in the direction of organic chemistry, can solve problems such as the selective synthesis of irrelevant compounds and pyrazine compounds that do not show
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0383] 3-Amino-5-chloro-6-(6-methoxy-3-pyridyl)-2-pyrazinecarbonitrile (1.35 g) was dissolved in dioxane (135 ml). To this solution was added phenylboronic acid (1.89 g) in water (27 ml) and Pd(PPh 3 ) 4 (179mg) and Na 2 CO 3 (2.19g). The reaction mixture was heated at 80°C for 2 hours and then at ambient temperature for 3 hours. The above mixture was partitioned between EtOAc and water. Separate the organic layer with aq.Na 2 CO 3 and brine, washed with MgSO 4 dry. The solvent was evaporated in vacuo to give an oily residue, which was purified by silica gel chromatography (EtOAc:n-Hexane=1:1, v / v) to give 3-amino-6-(6-methoxy-3-pyridyl)-5 -Phenyl-2-pyrazinecarbonitrile as a yellow powder which was crystallized from EtOAc (1.15 g).
[0384] 1 H-NMR (DMSO-d 6 δ): 3.81 (3H, s), 6.73 (1H, d, J = 8.6Hz), 7.35 (5H, s), 7.51 (2H, s), 7.54 (1H, dd, J = 2.4, 8.6Hz), 7.99 (1H, d, J = 2.4Hz)
[0385] MS (ESI + ): 304[M+H] + , 326[M+Na] +
[0386] IR(KBr): 3357, 3183, 2...
Embodiment 2
[0389] 3-Amino-6-(6-methoxy-3-pyridyl)-5-phenyl-2-pyrazinecarbonitrile (500 mg) was dissolved in dioxane (10 ml) and conc. HCl (5 ml). The solution was stirred at 80°C for 5 hours. The reaction mixture was cooled to 25-30°C and concentrated in vacuo to obtain a residue. Water was added to the residue and the pH of the aqueous mixture was adjusted to 6-7 with 1N NaOH. The precipitated crystals were collected by filtration and dried in vacuo to obtain 3-amino-6-(6-oxo-1,6-di-hydro-3-pyridyl)-5-phenyl-2-pyrazinecarboxamide (390 mg) .
[0390] 1 H-NMR (DMSO-d 6 δ): 6.16 (1H, d, J=9.4Hz), 7.26-7.70 (10H, m), 8.23 (1H, s), 11.66 (1H, s)
[0391] MS (ESI + ): 330[M+Na] +
[0392] MS (ESI - ): 306[M-H] -
[0393] IR (KBr): 3309, 1656, 1610, 1544, 1201cm -1
[0394] m.p.: 215-220°C (H 2 O)
Embodiment 3
[0396] 3-Amino-6-(6-oxo-1,6-dihydro-3-pyridyl)-5-phenyl-2-pyrazinecarboxamide (61.4 mg) was dissolved in DMF (1 ml). To this solution was added a solution of 1M MeI in DMF (0.22ml) and a solution of 0.1M t-BuOK in DMF (2.2ml). The mixture was stirred at 20-30°C for 2 hours. The reaction mixture was partitioned between EtOAc and water. Separate the organic layer. The aqueous layer was extracted with EtOAc. The combined organic solutions were washed with brine, washed with MgSO 4 dry. The solvent was evaporated to obtain an oily residue. The residue was purified by silica gel chromatography (EtOAc-EtOAc:MeOH=93:7 alone, v / v) to give 3-amino-6-(1-methyl-6-oxo-1,6-dihydro- 3-pyridyl)-5-phenyl-2-pyrazinecarboxamide which was crystallized from EtOAc (20 mg).
[0397] 1 H-NMR (DMSO-d 6 δ): 3.45 (3H, s), 6.12 (1H, d, J=9.4Hz), 6.97 (1H, dd, J=2.4, 9.4Hz), 7.41-7.62 (8H, m), 8.14 (1H, d , J=2.4Hz), 8.29(1H, s)
[0398] MS (ESI + ): 344[M+Na] +
[0399] IR (KBr): 3353, 166...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com