Method for synthesizing 16-dehydropregndiketonic alcohol acetic ester and its analogs
A technology of diketene alcohol acetate and its congeners, which is applied in the field of chemical synthesis of drugs, can solve the problems of expensive metal catalysts, environmental pollution, environmental pollution, etc., and achieve less strict temperature control requirements, environmental friendliness, and elimination of environmental pollution problem effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
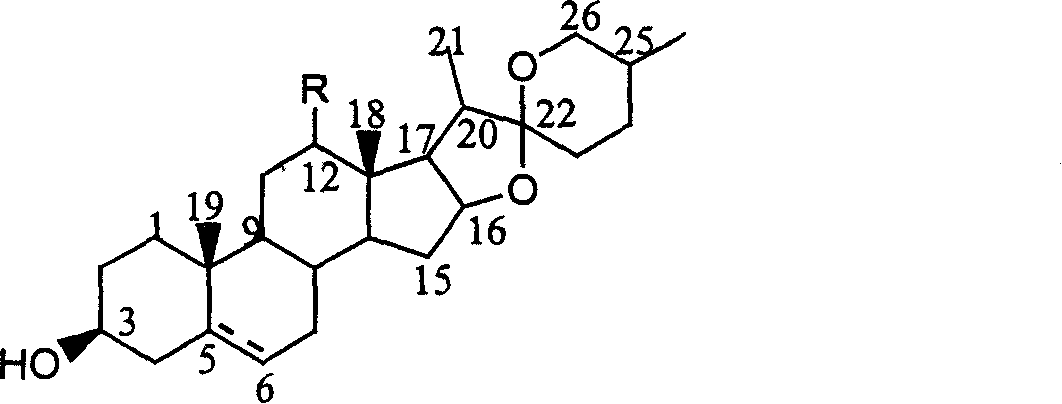
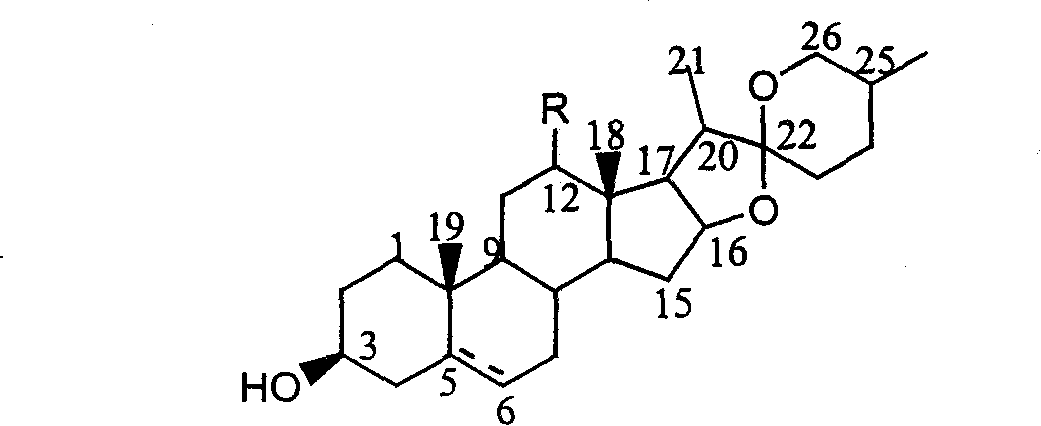
[0028] Oxidative degradation of semagenin to 5α-pregnene-12,20-diketone-3β-hydroxyacetate:
[0029] Dissolve 10 g (0.02 mol) of safranin acetate in 75 ml of acetic anhydride, add 2.6 g (0.022 mol) of pyridine hydrochloride, heat to reflux, and after the conversion of raw materials is complete (about 4 hours), distill under reduced pressure Go out most of acetic anhydride, under stirring, remaining mixture is poured into 100ml ice water, produces a large amount of yellow precipitates, and the precipitate filtered out is recrystallized with methanol, and the product obtained is pseudosacetin, and the productive rate is 70% (documentation value was 84%).
[0030] The light source used in the photooxidation reaction experiment is a medium-pressure mercury lamp, which is built in a cold jacket made of quartz, and a Pyrex glass photoreactor with a ventilation device is used. Take 2g (0.004mol) of pseudosemagenin in the light reaction vessel, and add the concentration of 1 × 10 -3 ...
Embodiment 2
[0032] Oxidative degradation of semagenin to 5α-pregnene-12,20-diketone-3β-hydroxyacetate:
[0033] The light source used in the photooxidation reaction experiment is a medium-pressure mercury lamp, which is built in a cold jacket made of quartz, and a Pyrex glass photoreactor with a ventilation device is used. Get 2g (0.004mol) pseudosemagenin synthesized in embodiment 1 in the photoreaction container, adding concentration is 2 * 10 -3 mol / L acetonitrile solution of hematoporphyrin in 20ml, then add 1ml (about 0.012mol) of pyridine and 1ml (about 0.01mol) of acetic anhydride, and pass through oxygen. The optical filter passes light with a wavelength of 380 to 600 nm. Use HPLC to track the reaction, stop the light immediately when the pseudosacenagenin is completely converted, evaporate the acetonitrile solvent under reduced pressure, then add 15ml of glacial acetic acid, heat and reflux for 3 hours, concentrate, dissolve in petroleum ether, wash with dilute alkaline water un...
Embodiment 3
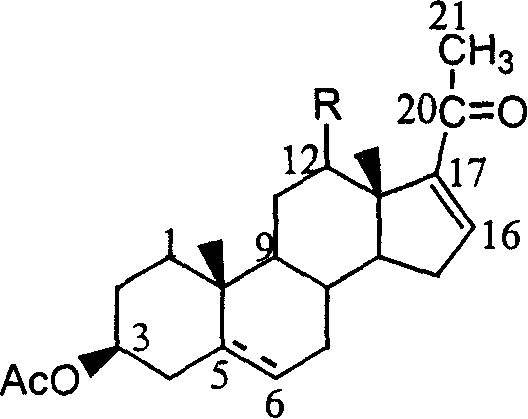
[0035] Oxidative degradation of diosgenin to 16-dehydropregnadienol acetate:
[0036] Take 20g (48.3mmol) of diosgenin in a 500ml three-necked bottle, add about 150ml of acetic anhydride, stir and reflux with nitrogen for one hour, stop heating, and distill out about 30ml of acetic anhydride solvent under reduced pressure after cooling down slightly. Add 6.5g (56.3mmol) of pyridine hydrochloride into the three-necked flask, and reflux at about 160°C for 5 hours to obtain a dark red solution. Distill under reduced pressure to remaining 30ml reaction mixture, this mixture is poured into 100ml ice water under agitation, produces a large amount of yellow precipitates, and the precipitate filtered out is recrystallized with methanol, and the resulting product is pseudodiosgenin, and vacuum drying gives 18g light yellow solid, The yield was 75%.
[0037] The light source used in the photooxidation reaction experiment is a medium-pressure mercury lamp, which is built in a cold jacke...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com