Methods for extracting a tooth
a tooth extraction and tooth technology, applied in the field of tooth extraction methods, can solve the problems of bone tissue being removed or damaged during the procedure, fluid seepage or weeping, and other forms of fluid loss that are typically present, and achieve the effect of accelerating the restoration of the extracted tooth and reducing the risk of tooth extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Defragmentation of Gingival Tissue In Vitro by Collagenase
[0111]A. Dimension Measurement Using a Millimetric Grid
[0112]Gingival tissue was chosen for the purpose of In vitro experiments, as it can be easily obtained at various sizes and has many common characteristics with PDL, where PDL is too thin and delicate for this kind of experiments.
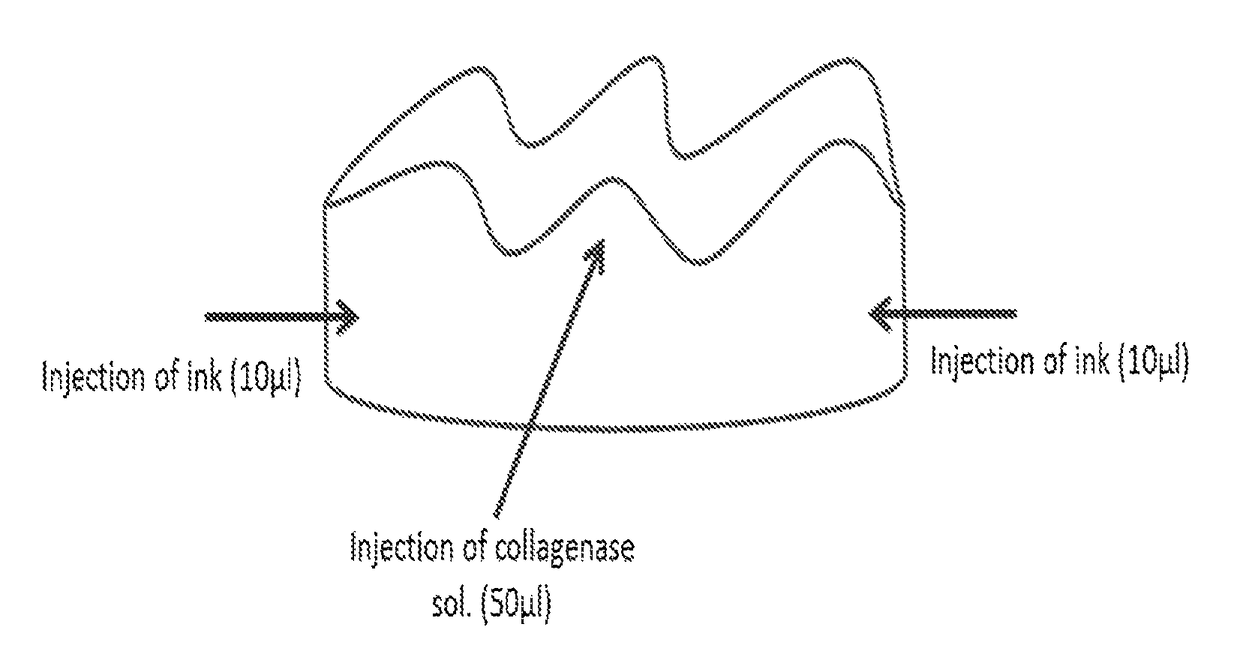
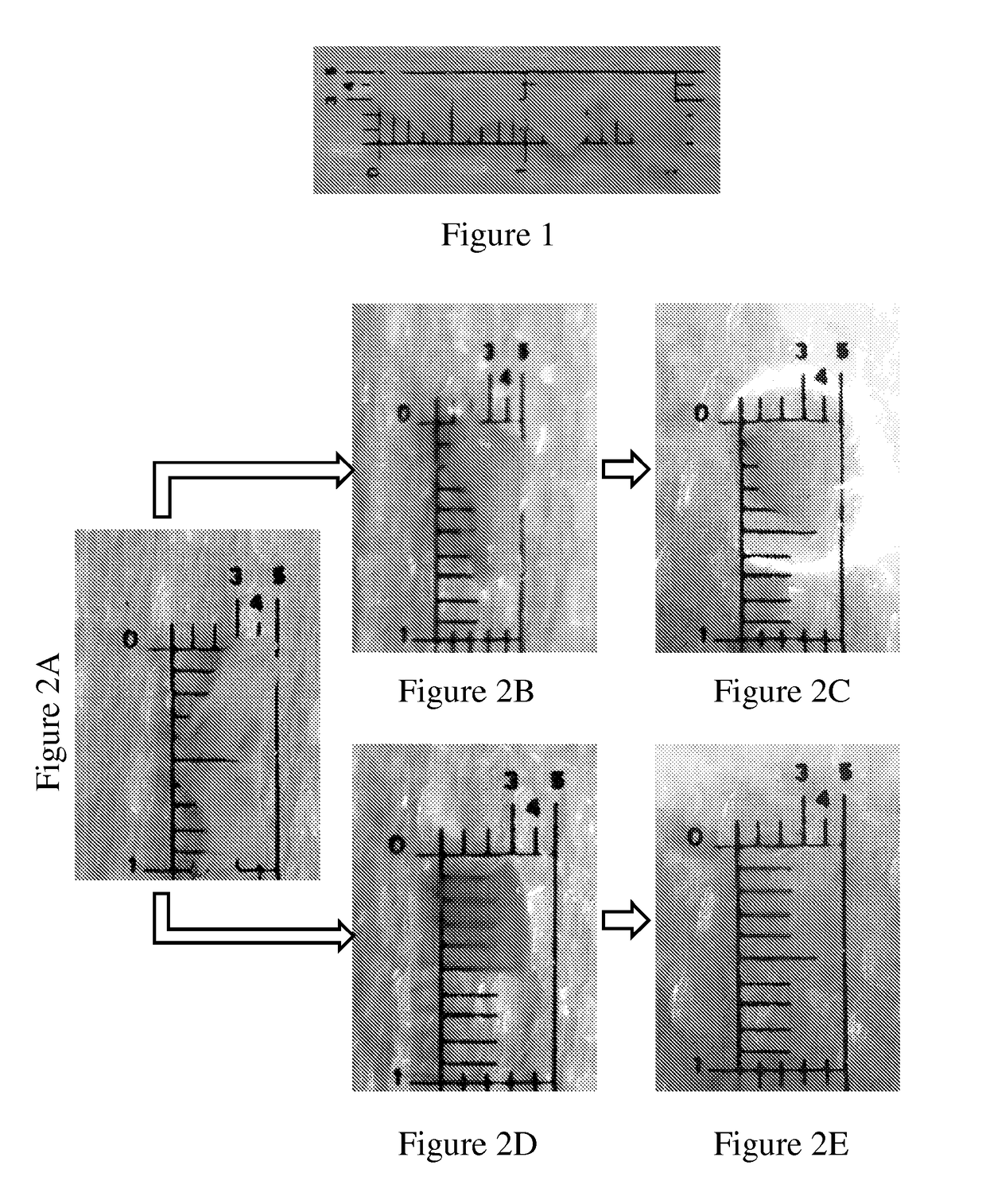
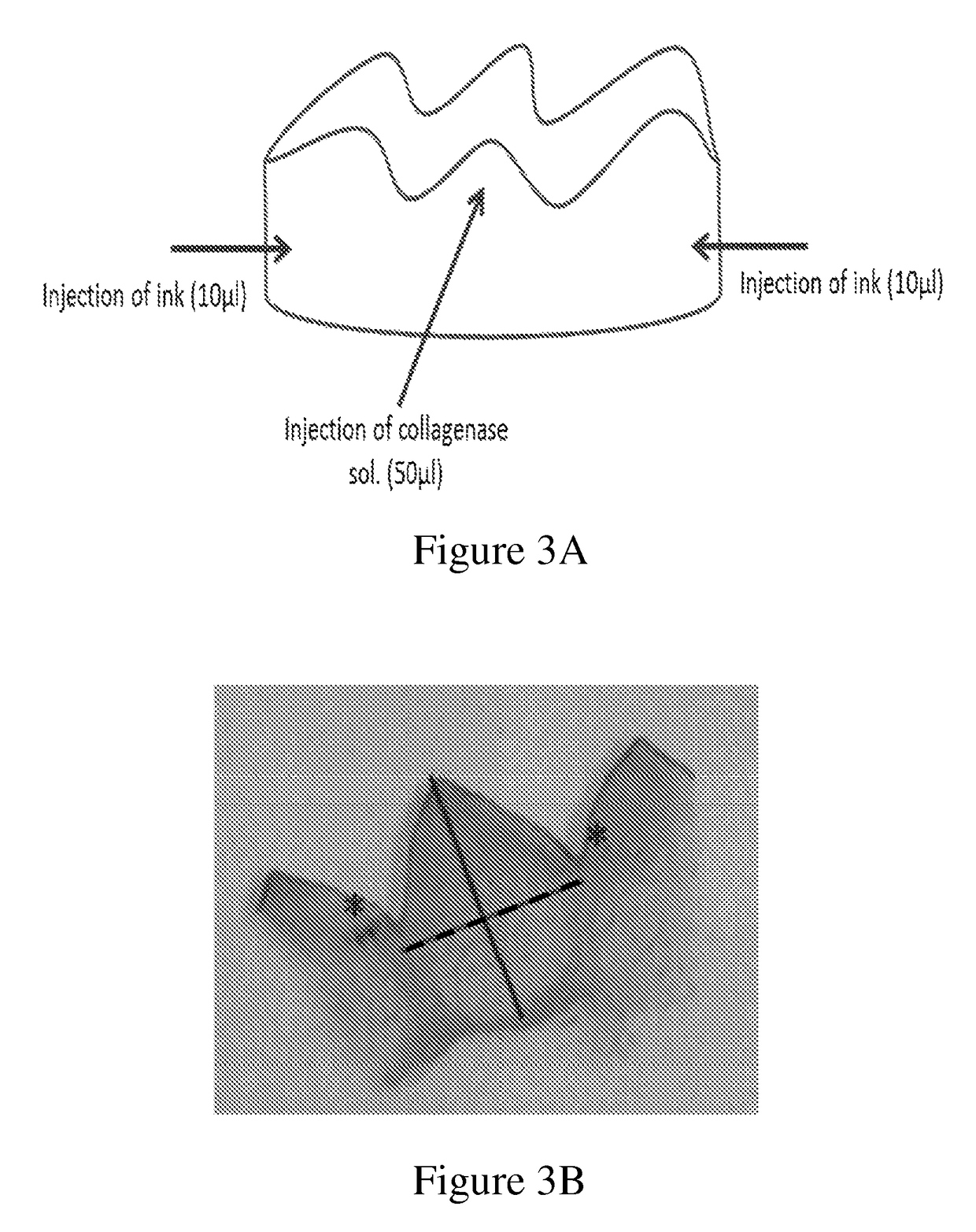
[0113]A band of gingival tissue, 20×3×0.3 mm3, was obtained from the diastema region of pig mandible within a few minutes following sacrifice. The band was sliced into four equal specimens about 5×3×0.3 mm3 each (FIG. 1 and FIG. 2A).
[0114]Two specimens were conditioned in 0.5 ml of phosphate-buffered saline (PBS) containing 5450 units / ml collagenase CLPSA solution (Worthington Biochemical Corporation, NJ, USA; FIGS. 2D and 2E) and two specimens were conditioned in 0.5 ml PBS (FIGS. 2B and 2C) and served as control. All four specimens were incubated in 37° C. water bath under gentle agitation for different time intervals.
[0115]The extent of tissue...
example 2
Assessment of the Intraligamental Injection Volume Ex-Vivo
[0126]Assessment was based on empirical findings regarding the length of the teeth to be treated and the width of the periodontal ligament at specific sections. This data was used to calculate the estimated PDL volume. This estimation was then reevaluated clinically.
[0127]Indian ink was injected into the PDL of mandibular 3rd incisor and 1st premolar, at 6 points. The end point was determined by the injection volume causing dye leakage. The optimal volume was determined as 20 μl at each of the 6 points of injection.
example 3
PDL Defragmentation Following Collagenase Injection
[0128]In order to evaluate PDL structure rather than gingival structure, mandibular 3rd incisor and 1st premolar bilaterally and the tissue surrounding same, were subjected to enzyme injection (20 μl at 6 points) at different concentrations (2,500; 5,000; 7,500 or 10,000 units / ml). Two hours post injection the mandibles were cut and trimmed around the relevant tooth, leaving a tooth embedded in the bone socket, then fixed in formalin. Specimens were decalcified for 5-6 days, then trimmed (FIG. 5), embedded in paraffin and sectioned along the tooth's long axis.
[0129]The sections were stained with Hematoxylin & Eosin and Picrosirius Red and transferred to histopathological examination in order to test PDL collagen fibers breakdown at different sections of the root and integrity of surrounding tissues structures (alveolar bone proper). Specimen that were injected with PBS or not injected served as control.
[0130]PDL Defragmentation was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass ratio | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com