Method for producing solid electrolyte having argyrodite type crystal structure
a technology of argyrodite and crystal structure, which is applied in the direction of electrolytes, cell components, electrical equipment, etc., can solve the problems of inability to produce batteries, short circuits, and inability to obtain fine solid electrolyte suitable for solid electrolyte layers having a thickness on the order of submicron,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Production of Lithium Sulfide (Li2S)
[0122]Li2S was produced and purified as described below.
[0123]As a water-insoluble medium, toluene (manufactured by Sumitomo Corporation) was dehydrated, and 303.8 kg of toluene having a moisture content of 100 ppm, which was measured by a Karl Fisher moisture meter, was added to a 500 L reaction kiln made of stainless steel under a nitrogen stream. Then, 33.8 kg of anhydrous lithium hydroxide (manufactured by Honjo Chemical Co., Ltd.) was addition, and the mixture was kept at 95° C. while being stirred at 131 rpm by a twin star stirring blade.
[0124]Hydrogen sulfide (manufactured by Sumitomo Seika Co., Ltd.) was blown into the slurry at a feed rate of 100 L / min, and the temperature was raised to 104° C. An azeotropic gas of water and toluene was continuously discharged from the reaction kiln. This azeotropic gas was condensed by an out-of-system condenser to achieve dehydration. In the meantime, the same amount of toluene as distilling toluene was...
example 1
(A) Pulverizing Step
[0128]Li2S obtained in Production Example 1 was pulverized in a nitrogen atmosphere using a pin mill (100UPZ manufactured by Hosokawa Micron Co., Ltd.) having a fixed quantity machine. The addition rate was 80 g / min, and the rotation speed of the disk was 18000 rpm.
[0129]Similarly, P2S5 (manufactured by Thermophos, laser diffraction D50=125 μm), LiBr (manufactured by Honjo Chemical, laser diffraction D50=38 μm), and LiCl (manufactured by Sigma Aldrich, laser diffraction D50=308 μm) were each pulverized by a pin mill. The addition rate of P2S5 was 140 g / min, the addition rate of LiBr was 230 g / min, and the addition rate of LiCl was 250 g / min. The rotation speed of each disk was 18000 rpm.
[0130]The laser diffraction D50 of Li2S after pulverizing was 7.7 μm, the laser diffraction D50 of P2S5 after pulverizing was 8.7 μm, the laser diffraction D50 of LiBr after pulverizing was 5.0 μm, and the laser diffraction D50 of LiCl after pulverizing was 10 μm, respectively.
(B)...
example 2
[0141]In Example 1(B), the slurry after treatment with the bead mill was used in the heat-treating step without drying. The slurry was put into an autoclave and heat-treated at 180° C. for 2 hours. Except for the foregoing, a solid electrolyte was produced and evaluated in the same manner as in Example 1. The results are shown in Tables 1 and 2.
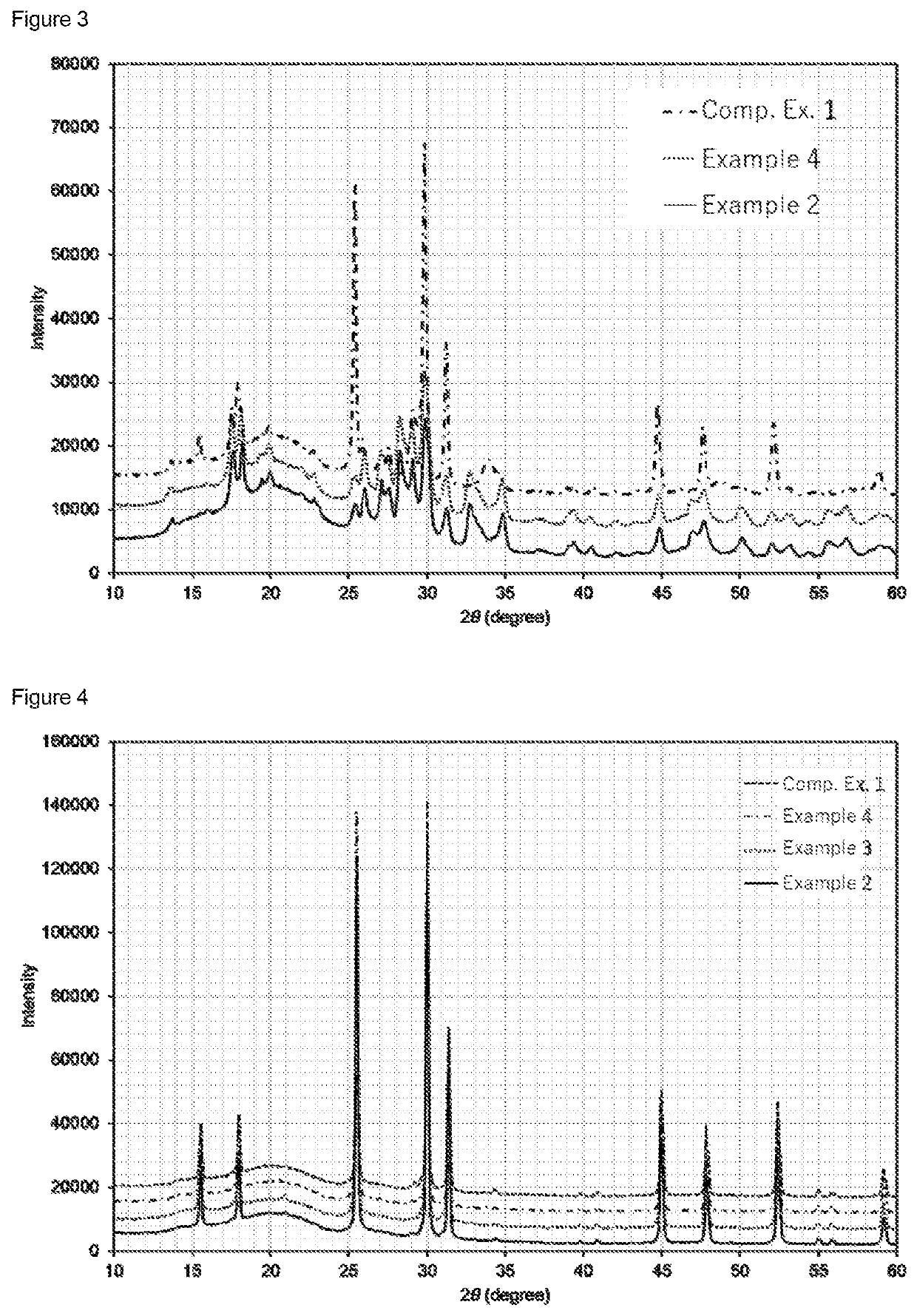
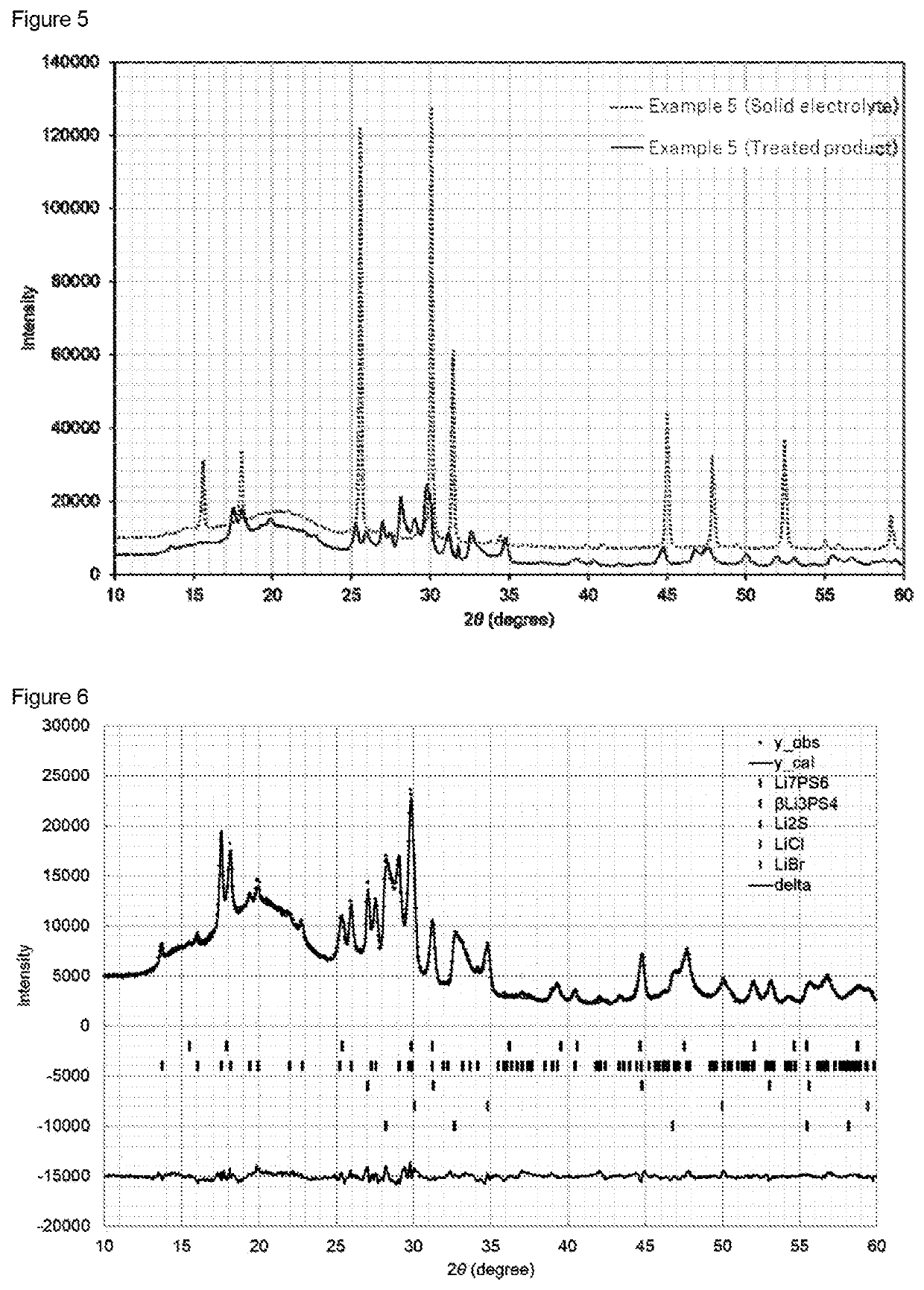
[0142]The XRD pattern of the treated product is shown in FIG. 3. The XRD pattern of the solid electrolyte is shown in FIG. 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com