Method for preparing anode material carbon coated lithium titanate for lithium ion power batteries
A carbon-coated lithium titanate, power battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of poor particle size electron conductivity, poor battery rate performance, etc., to improve electronic conductivity and power. performance, the effect of improving current density and power density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
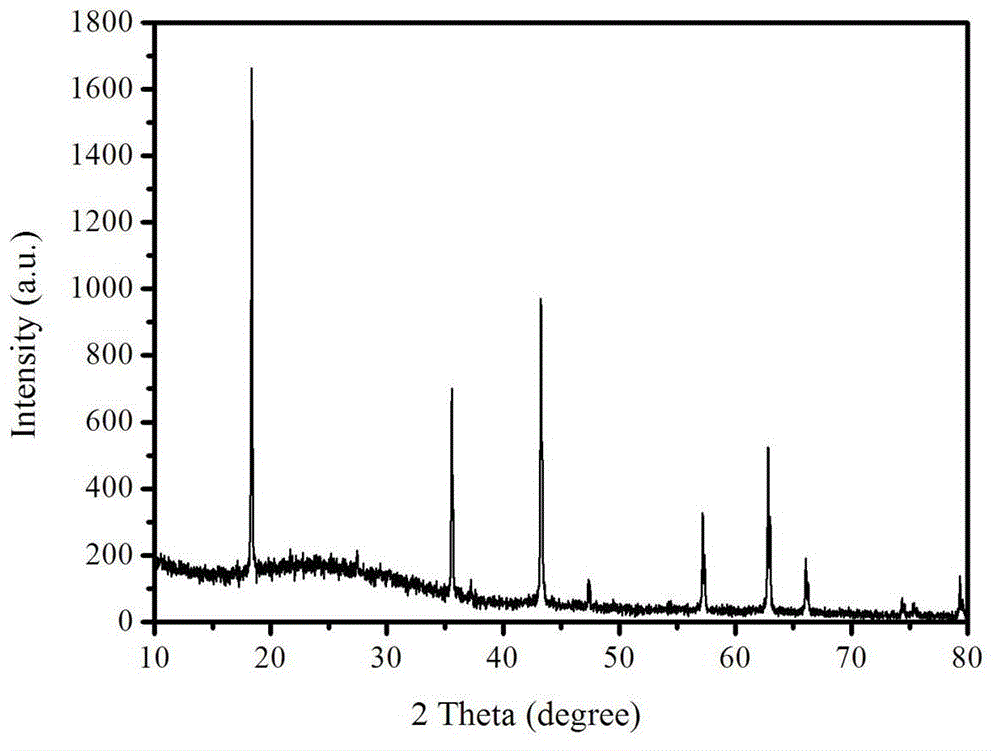
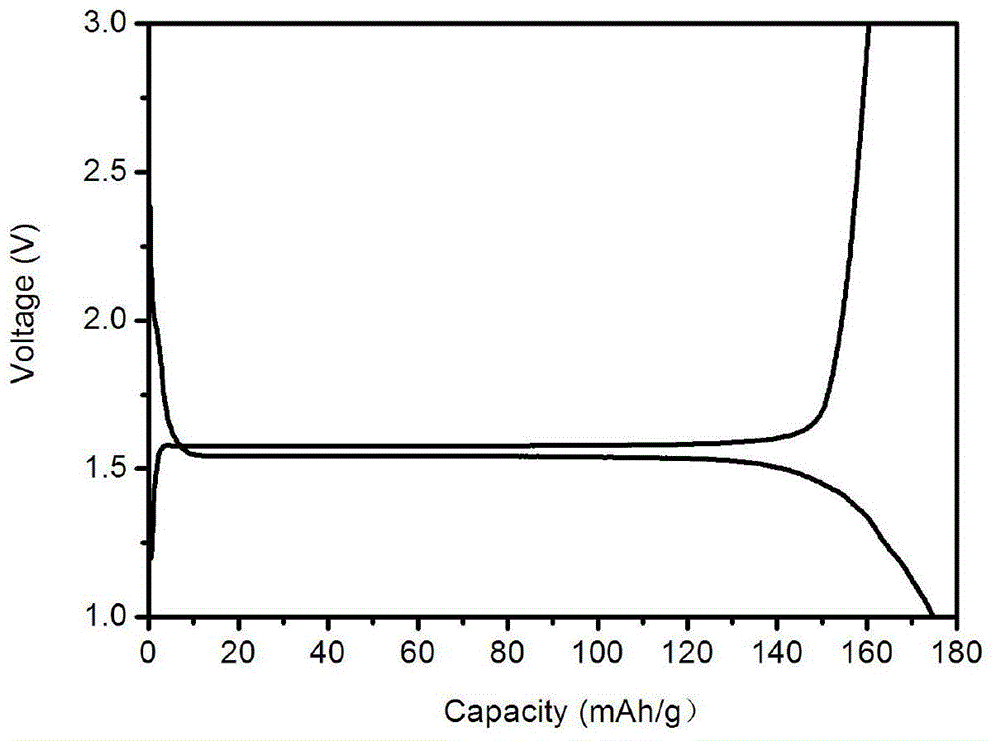
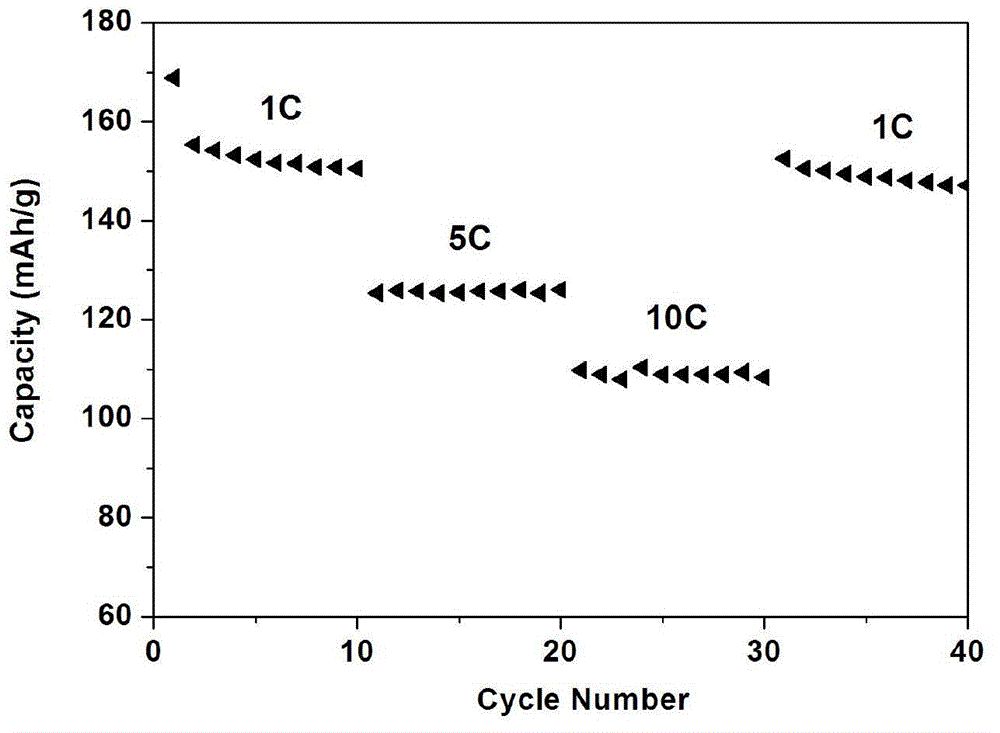
[0022] Example 1: Weigh 3.0g sucrose and 6.0g titanium dioxide respectively, place them in a 250mL beaker, add 80mL absolute ethanol, stir for 6h, and place them in an oven at 80℃ for drying; the dried sucrose and titanium dioxide mixture is Sintering in nitrogen at 600°C, holding time for 5 hours, and naturally lowering to room temperature, to obtain carbon-coated nano titanium dioxide; the mixture of lithium carbonate and lithium oxalate (the mass fraction of lithium oxalate is 1%) and titanium dioxide, according to Li :Ti=0.8:1 molar ratio for compounding, ball milling in acetone medium, drying, and sintering in nitrogen at 800°C for 10 hours to obtain carbon-coated lithium titanate powder.
Embodiment 2
[0023] Example 2: Weigh 0.6g sucrose and 2.0g titanium dioxide respectively, place them in a 250mL beaker, add 80mL absolute ethanol, stir for 6h, and place them in an oven at 80℃ for drying; the dried sucrose and titanium dioxide mixture is Sintering in nitrogen at 600°C, holding time for 5 hours, and naturally lowering to room temperature, to obtain carbon-coated nano titanium dioxide; the mixture of lithium carbonate and lithium oxalate (the mass fraction of lithium oxalate is 1%) and titanium dioxide, according to Li :Ti=0.8:1 molar ratio for compounding, ball milling in acetone medium, drying, and sintering in nitrogen at 800°C for 10 hours to obtain carbon-coated lithium titanate powder.
Embodiment 3
[0024] Example 3: Weigh 2.0g glucose and 4.0g titanium dioxide respectively, put them in a 150mL beaker, add 60mL absolute ethanol, stir for 6h, and place them in an oven at 80℃ for drying; the dried glucose and titanium dioxide mixture is Sintering in nitrogen at 600°C, holding time for 5 hours, and naturally lowering to room temperature to obtain carbon-coated nano titanium dioxide; the mixture of lithium acetate and lithium oxalate (the mass fraction of lithium oxalate is 1%) and titanium dioxide, according to Li :Ti=0.8:1 molar ratio for compounding, ball milling in acetone medium, drying, and sintering in nitrogen at 800°C for 10 hours to obtain carbon-coated lithium titanate powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com