Treatments for neurogenetic disorders, impulse control disorders, and wound healing
a neurogenetic disorder and impulse control technology, applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of affecting the health of affected individuals, their families, society, and their families, and causing premature death or lifelong disability with significant psychological and economic hardship, and affecting the quality of life of affected individuals, etc., to achieve the effect of promoting wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effects of topiramate on impulsivity and cognitive functioning
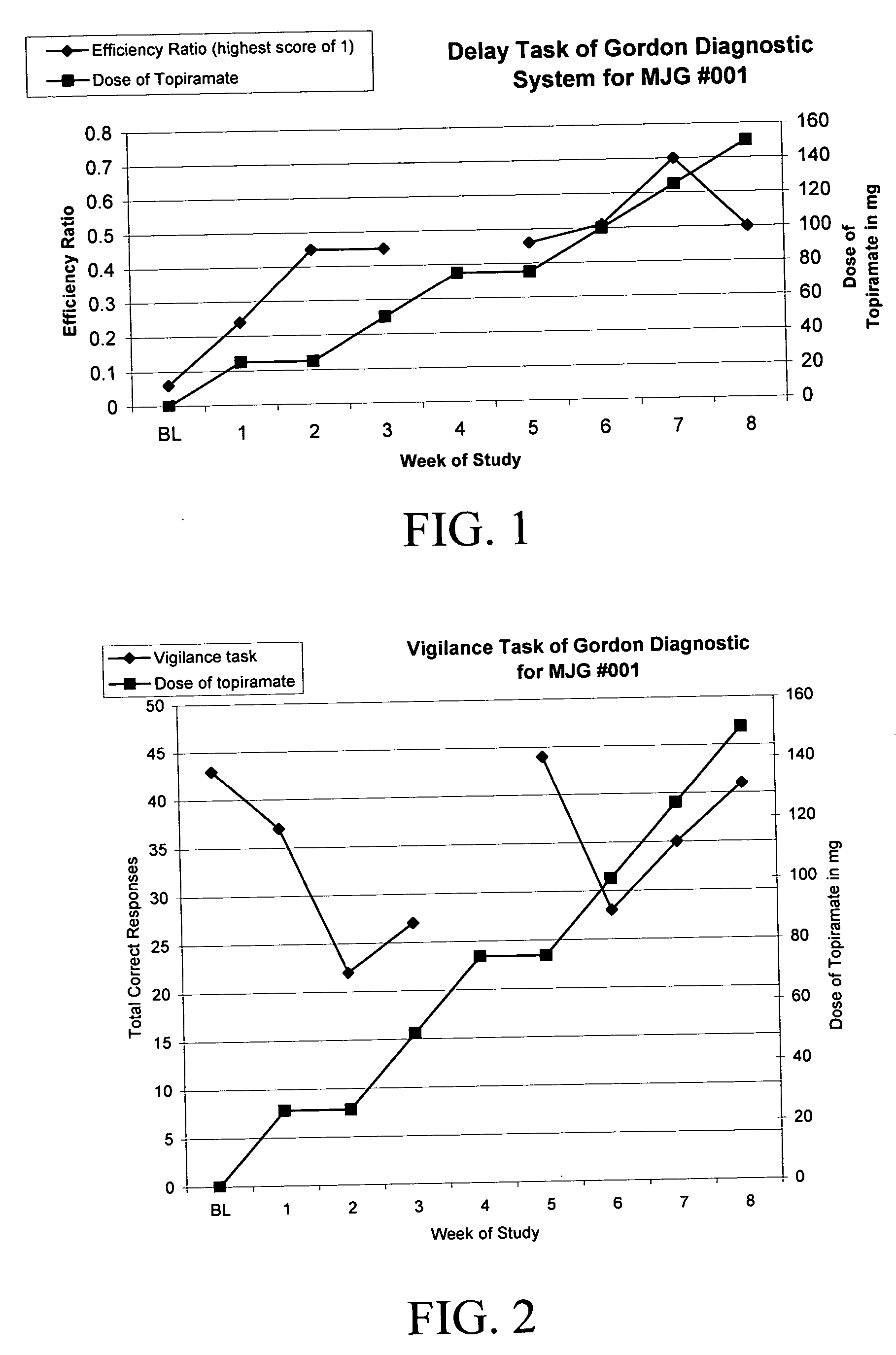
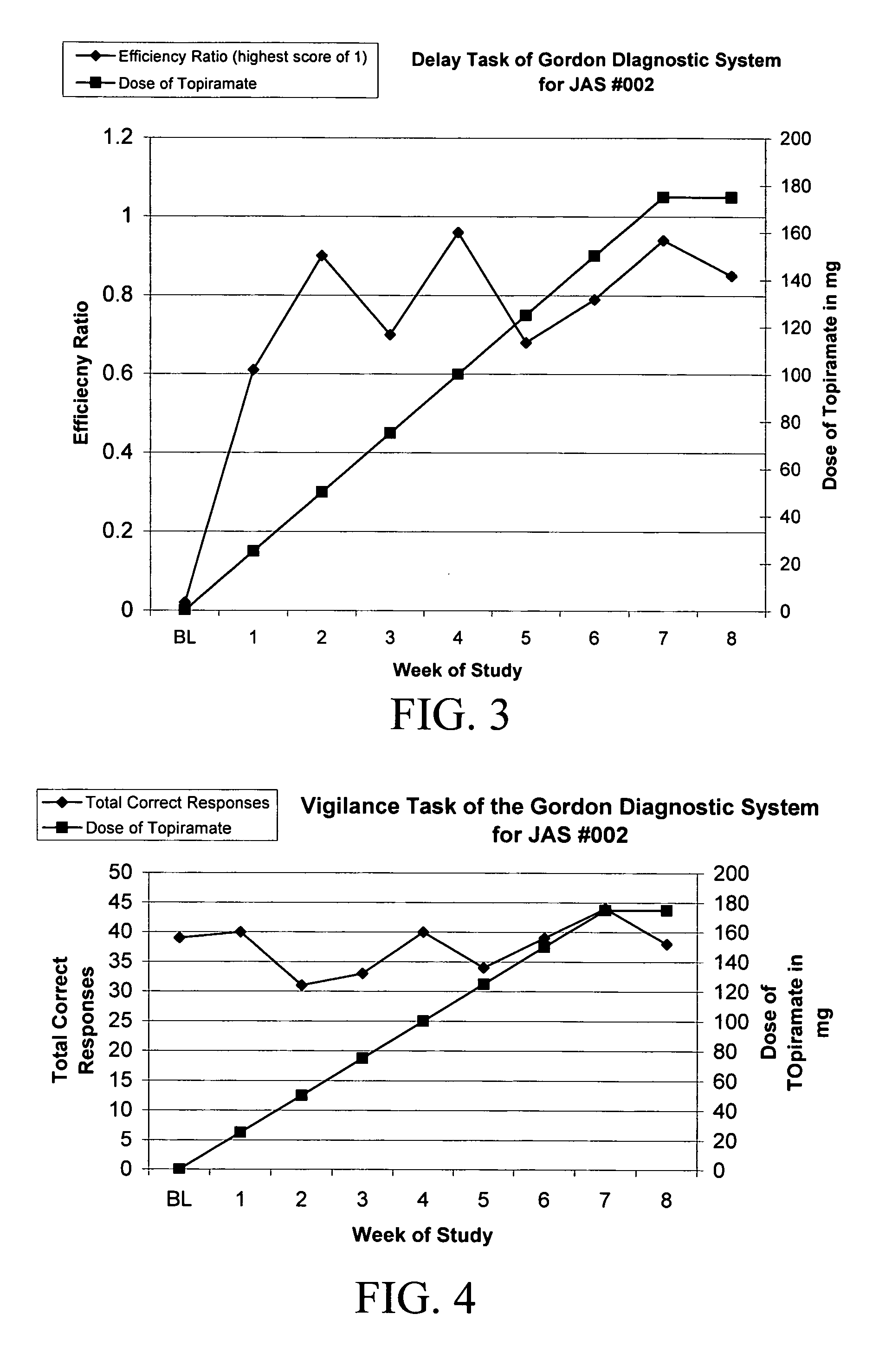
[0071] There are no reports of topiramate being utilized in PWS for any behavior. Measurements of attention, concentration, and impulsivity were assessed by the Delay and Vigilance tasks of the Gordon Diagnostic System (GDS; Gordon, M.; McClure, F. D.; & Aylward, G. P. (1996) Gordon Diagnostic System, Interpretive Guide (Third Edition); Dewitt, N.Y.: Gordon Systems, Inc.), a mechanized evaluator of cognitive functions, including attention and concentration. The GDS was originally developed, and most commonly used, to measure aspects of Attention-Deficit / Hyperactivity Disorder (ADHD) (previously called Attention-Deficit Disorder). One of the important components of the Vigilance test is the ability of an individual to have sustained attention, and the Delay task in part measures the subjects' ability to concentrate and focus on hitting a button at appropriate time intervals and delay impulsive behavioral responses.
[0072]...
example 2
Effect of topiramate on pathologic skin picking (PSP)
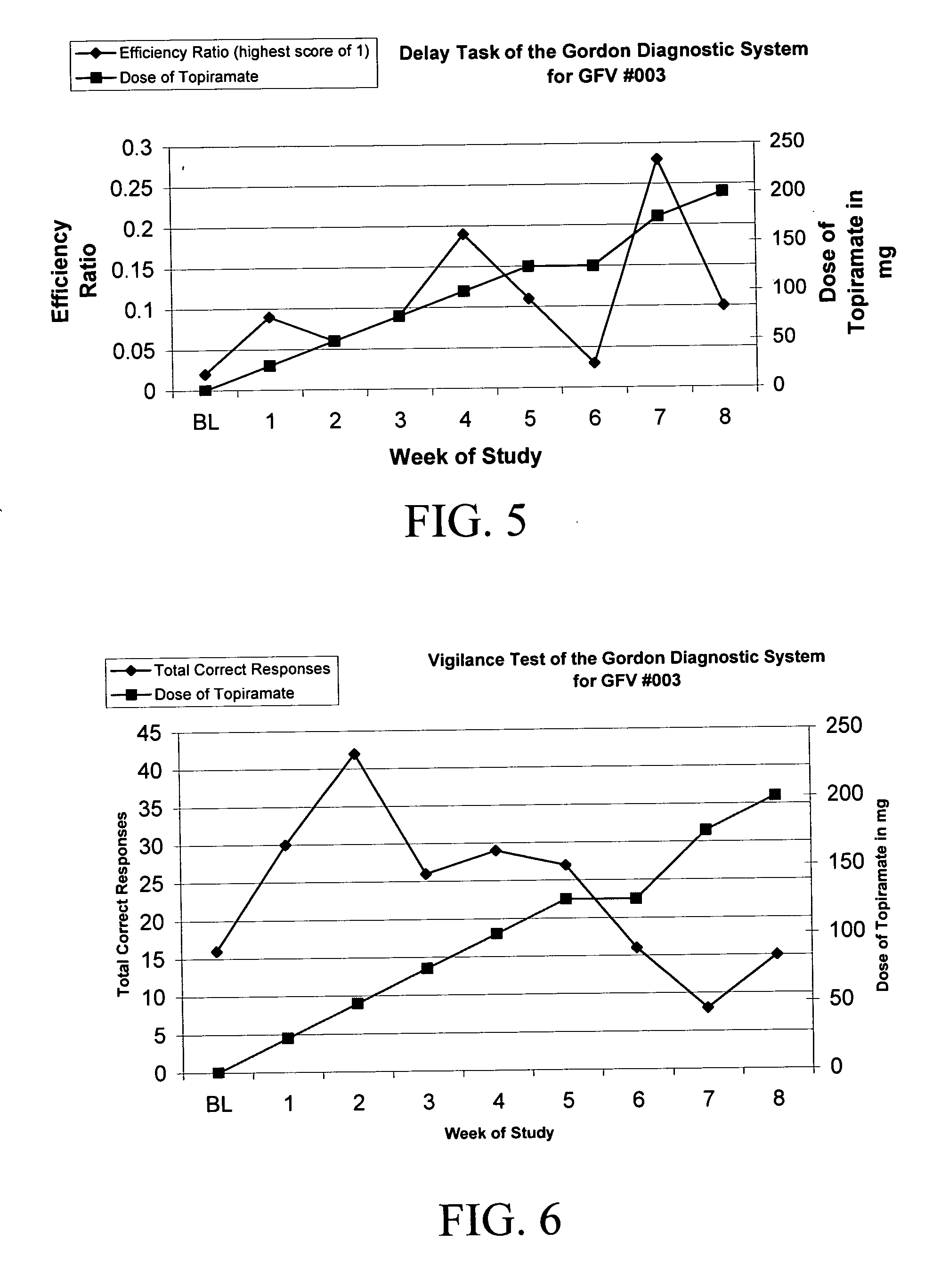
[0078] There are no reports of topiramate being utilized in PWS for any behavior. Patient #3 (GFV-003) also has pathologic skin picking (PSP) in addition to food seeking behavior. Patient #3 has a chronic large lesion on his lower left arm. Within one week of topiramate (25 mg / day), he had decreased skin picking and showed healing of this lesion on his left arm. By the 4th week on topiramate (at 100 mg / day), the lesion on his lower left arm had completely healed over. The progression of wound healing is provided in FIGS. 9A-D and 12A-B.
[0079] Patient #1 (MJG-001) has pathologic skin picking (PSP) in addition to food seeking behavior. Unexpectedly and serendipitously, it was observed that several large lesions (where she skin picks) on her right arm, legs, and lips were clearing up quickly (within 5 days) after topiramate was initiated at 25 mg / day. Furthermore, the patient has continued to do well in terms of skin clearing. For ...
example 3
Effects of Topiramate on patients with Prader-Willi Syndrome
[0081] In an 8-week, open-label, flexible-dose (maximum 350 mg / day) study to evaluate the efficacy and safety of topiramate in PWS adults, weekly evaluations were performed that included scales for stereotypical behavior (Stereotypy Checklist, Y-BOCS checklist), aberrant behavior (Aberrant Behavior Scale {ABS}, Severity of Symptoms Scale, Self-Injury and Self-Restraint Checklist) and cognitive functioning (Gordon Diagnostic, Controlled Oral Word Association Test, Semantic Naming test). Subject safety measures were also performed (e.g., monitoring blood pressure). Appetite was assessed for one hour at four time points during the trial. Measurements were made by investigator observation of the subject with free access to low calorie food and a visual analogue scale before and after observation.
[0082] Eight subjects (19-36 years of age; 4 males and 4 females) entered the trial and six have completed the treatment regimen. A ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight loss | aaaaa | aaaaa |
| weight loss | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap