Efficient methods for producing antimicrobial cationic peptides in host cells
a cationic peptide, host cell technology, applied in the direction of peptides, peptide sources, applications, etc., can solve the problems of low cost, loss of potassium ions, and decrease of cytoplasmic atp, and achieve the effect of mass-production methods, low cost, and low cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Plasmids PET21CBD-X(B) AND PET21CBD96
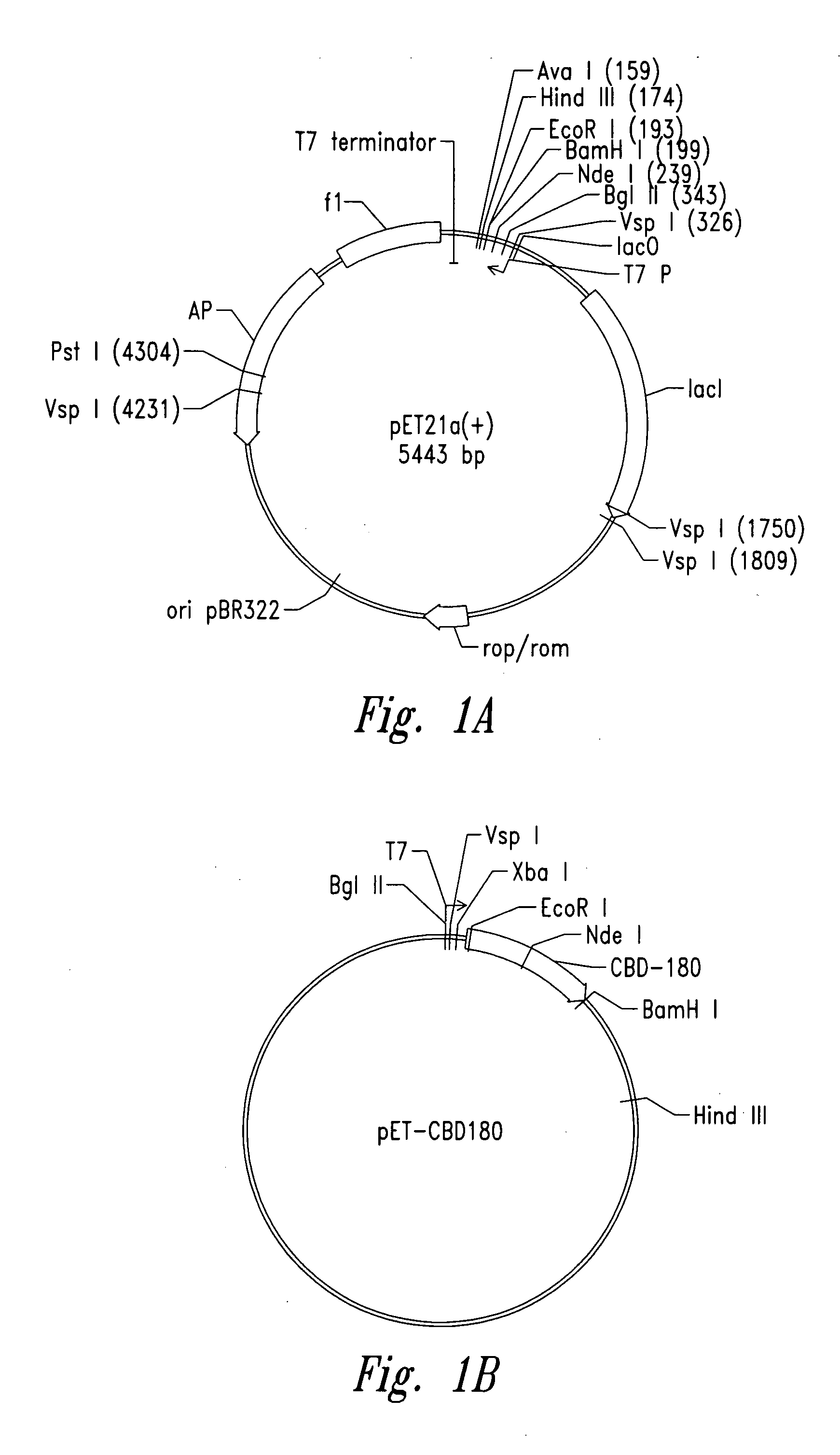
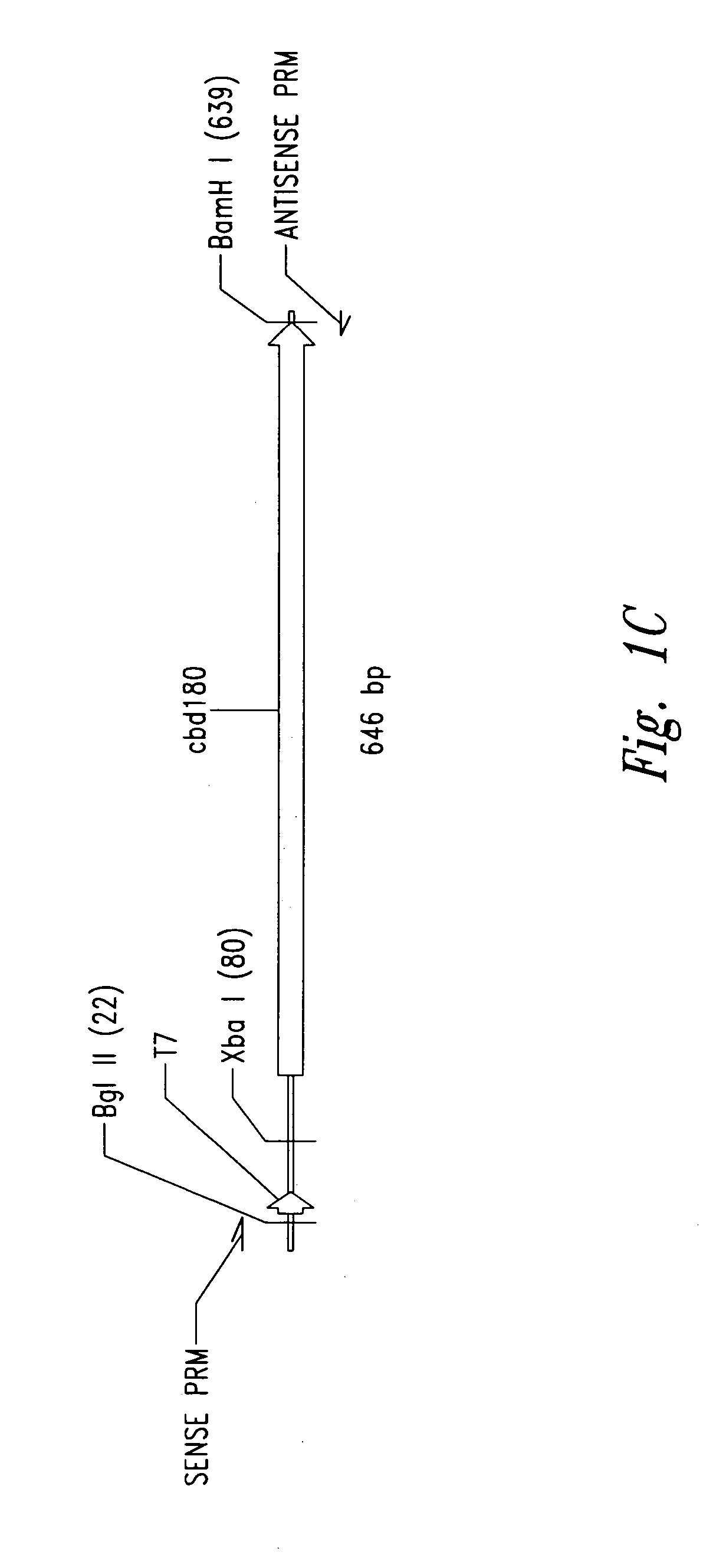
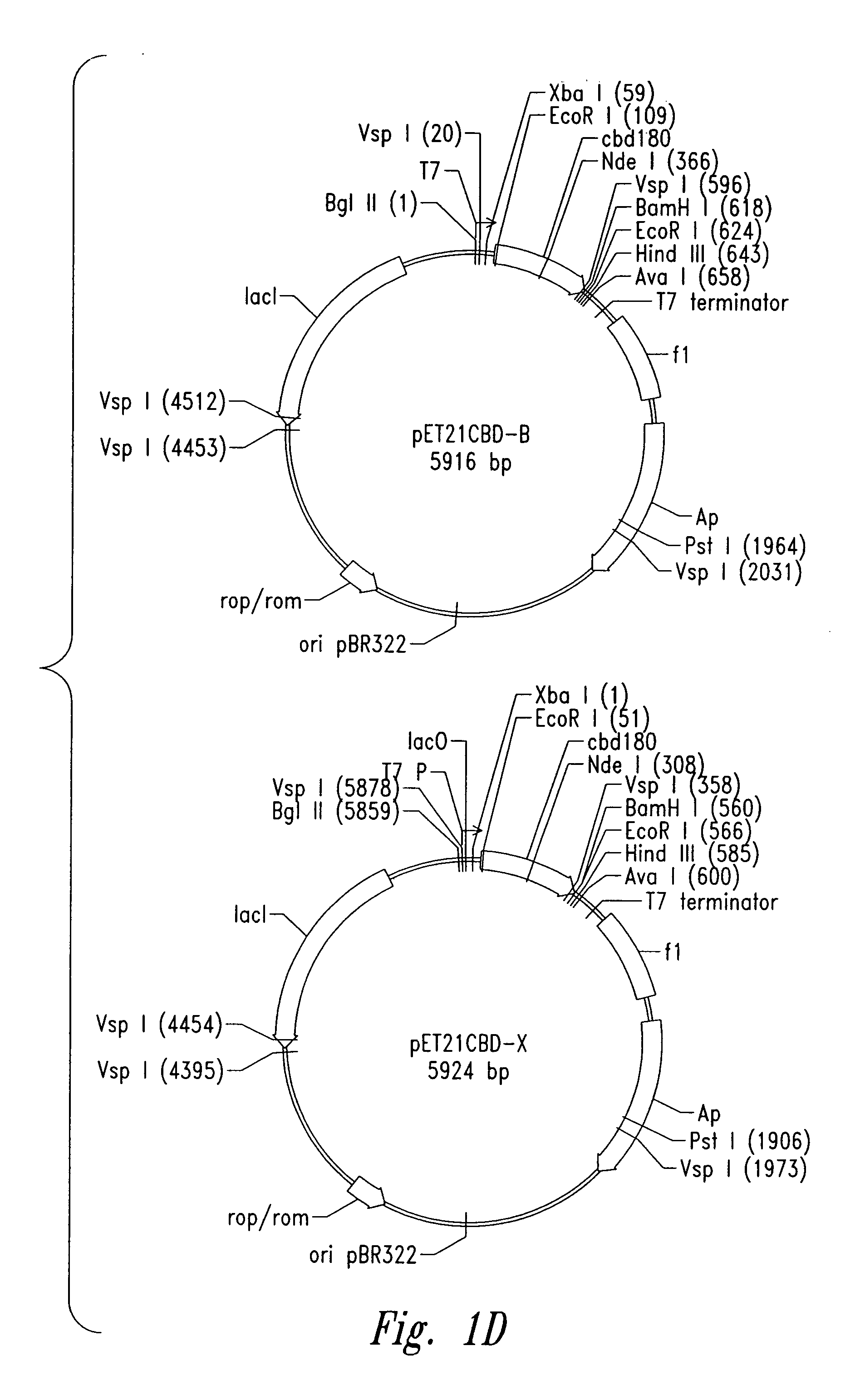
[0104] Plasmid vector pET21a(+) (Novagen Corporation, USA), a T7 expression plasmid, was used as the core plasmid for all expression systems (FIGS. 1A and 10). The cellulose binding domain (CBD) from Clostridium cellulovorans was selected as a carrier protein for expression of antibacterial cationic peptides. Plasmid pET-CBD180 (Shpigel et al., supra) was used as the starting material (FIGS. 1B and 10). Restriction enzymes except VspI and NsiI (Promega Corporation, USA), T4 DNA ligase and Taq polymerase were purchased from Pharmacia Biotech. The relevant part of CBD, including the T7 promoter of pET-CBD180, was amplified by PCR using 25 pmol each of each of the primers GCGT CCGG CGTA GAGG ATCG (SEQ ID NO:1) and CCGG GATC CAAT GTTG CAGA AGT AG (SEQ ID NO:2), 2 U of Taq DNA polymerase, corresponding reaction buffer (10× PCR reaction buffer: 500 mM KCl, 15 mM MgCl2, 100 mM Tris-HCl, pH 9), 0.2 mM dNTPs (dATP, dGTP, dTTP and dCTP, Ph...
example 2
Construction and Expression of CBD—MBI-11 Fusions
[0106] Sequences encoding all cationic peptides were designed from modified indolicidin, a natural anti-microbial peptide. Plasmids pET21CBD-X and pET21CBD96 (0.25 μg each) were digested with 2 U of BamHI and 2 U of HindIII in 1.5× OPA in 50 μl reactions at 37° C. for 1 hour. In the same way, a fragment encoding MBI-11 was digested (Example 4) using about 1 μg of DNA and 25U of BamHI and HindIII each in a 100 μL reaction. Both reactions were stopped by phenol / CHCl3 (Sigma-Aldrich Canada Ltd.) extraction and ethanol precipitation. The resultant DNAs of each vector and MBI-11 insert were dissolved in 8 μl of water and mixed, then 2 μl of 10 mM ATP, 2 μl of 10× OPA and 2 U of T4 DNA ligase were added and ligation reactions were incubated at 10° C. for 1 hour. Then 2 μl of each ligation mixture was used to electroporate 40 μl of E. coli XL1 Blue using sterile Gene Pulser cuvettes (0.2 cm electrode gap) and Gene Pulser electroporator appa...
example 3
Expression of CBD Fused Polycationic Peptide Tandem Domains
[0107] This experiment was designed to test how many peptide genes in tandem can be fused to a carrier protein and expressed. It was necessary to create two DNA fragments encoding MBI-11, one for polymerization by DNA cloning and another one as the last gene in the tandem. Therefore, the original DNA fragment encoding MBI-11 peptide with COOH end was modified in order to create the last gene in tandem (Example 4) and a new gene was designed for a specific cloning procedure, which allowed construction of multiple tandem peptide genes fused to CBD180 or CBD96 carrier proteins genes (Example 4). The cloning procedure resulted in addition of an extra isoleucine to the MBI-11 tandem sequences. Therefore in order to produce identical peptide molecules, an isoleucine codon was also added to the last gene sequence. CNBr will be used to cleave the peptide from fusion proteins, which means that peptide molecules would have a homoseri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com