Methods and compositions for producing germ cells from bone marrow derived germline stem cells
a technology of germ cells and compositions, which is applied in the direction of drug compositions, non-embryonic pluripotent stem cells, sexual disorders, etc., can solve the problems of failure to provide evidence of germinal epithelium and the inability of bone marrow derived cells to successfully repopulate, etc., to enhance or restore fertility, restore gonadal function, and replenish or expand germ cell reserves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Extra-Ovarian Female Germline Progenitor Cell Reservoirs
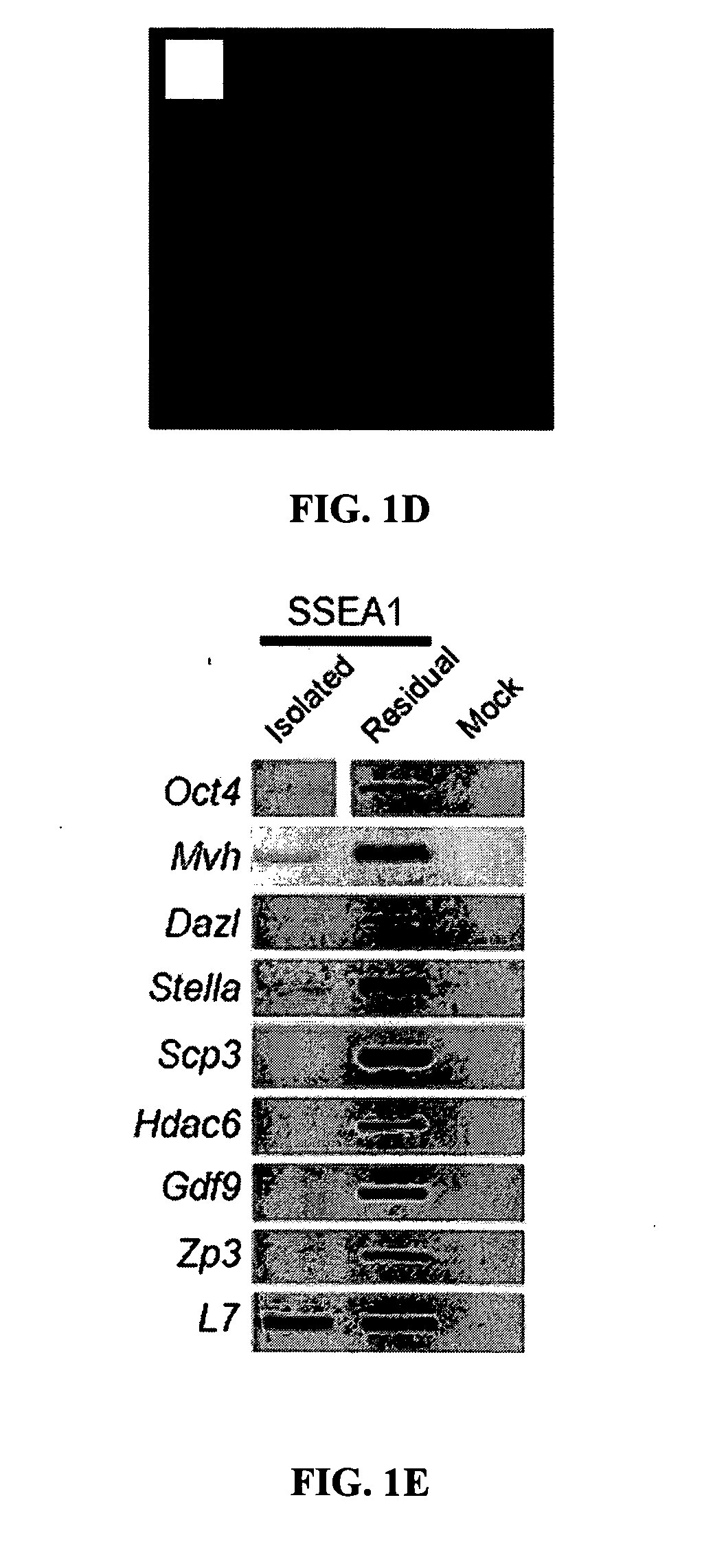
[0213] The restricted pattern of SSEA1 expression in the adult mouse ovary (FIG. 1a, b) suggested that the number of germline stem cells / progenitors thereof is relatively small. However, this would be incongruous with recent studies indicating that germline stem cells must offset an extremely robust rate of oocyte death for the gonads to remain functional throughout reproductive life (Johnson, J. et al (2004) Nature 428, 145-150) as well as with the ability of adult mouse ovaries to rapidly generate hundreds of new primordial oocyte-containing follicles. For details, see U.S. Application Ser. No. ______, filed on May 17, 2005 as Attorney Docket No. 51588-62054, the contents of which are herein incorporated by reference. Accordingly, the possibility that a larger germline stem cell reservoir exists somewhere outside of the ovaries was considered. The first clue in this regard was provided by the location of SSEA1+ cells in the ...
example 2
Bone Marrow Transplantation Reverses Pathological Ovarian Failure
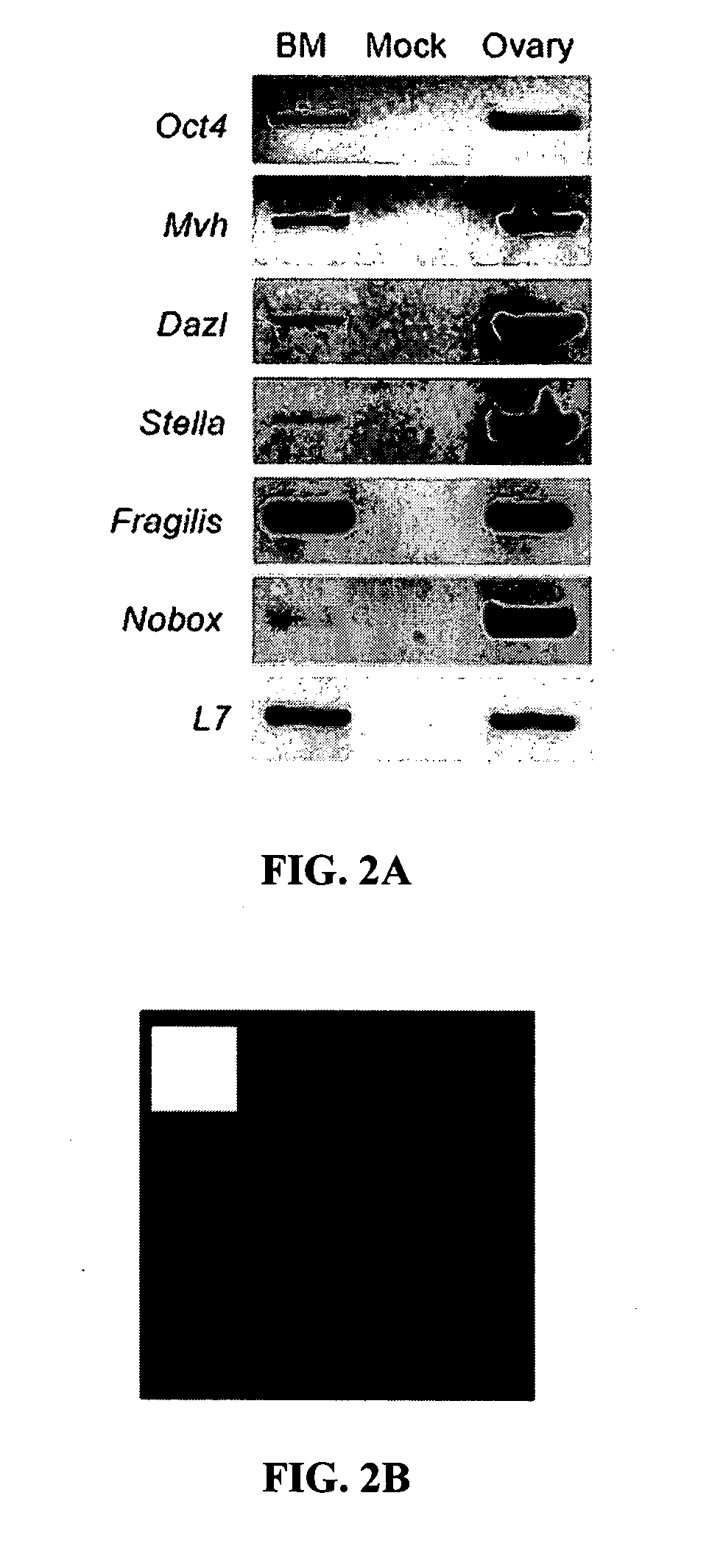
[0222] To assess the functional capacity of bone marrow-derived germ cells to produce new oocytes, bone marrow was isolated from adult wild-type female mice and transplanted using standard procedures into recipient adult females sterilized by treatment with a combination of cyclophosphamide and busulphan to destroy the existing pre- and post-meiotic germ cell pools prior to BMT.
[0223] Bone marrow was harvested from adult (6-10 weeks of age) wild-type C57BL / 6 female mice on the day of transplantation, and 2-5×107 cells were injected intravenously via the tail vein into recipients using standard procedures. To prepare recipients, female mice received 0.5 mg anti-CD4 antibody (GK1.5) (Dialynas, D. P. et al. (1984) J. Immunol. 131, 2445-2451) and 1 mg anti-CD8 antibody (2.43) (Sarmiento, M. et al. (1980) J. Immunol. 125, 2665-2672) one week prior to a second injection of each antibody along with 120 mg kg−1 cyclophospham...
example 3
Bone Marrow Transplantation Rescues Oocyte Production in Atm Mutants
[0226] Atm− / − (homozygous null) mice, created by targeted inactivation of the Atm gene, display many of the hallmarks of the Ataxia-telangiectasia syndrome in humans, including growth retardation, defects in T lymphocyte maturation and infertility (Bagley et al. (2004) Blood 12: 1). Atm-deficient male and female mice have been shown to be infertile due to the complete loss of the production of mature gametes, i.e., spermatozoa and oocytes (Barlow, C. et al. (1996) Cell 86: 159). These gametogenesis defects in mutant mice lacking Atm result from apoptosis and degeneration of the developing gametes that exhibit aberrant early stages of meiosis, detected as early as the leptotene stage (Barlow, C. et al. (1998) Development 125: 4007). Ovaries from Atm-deficient females were shown to be completely barren of oocytes and follicles by at least 11 days of age postpartum (Barlow, C. et al. (1998) Development 125: 4007).
[02...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com