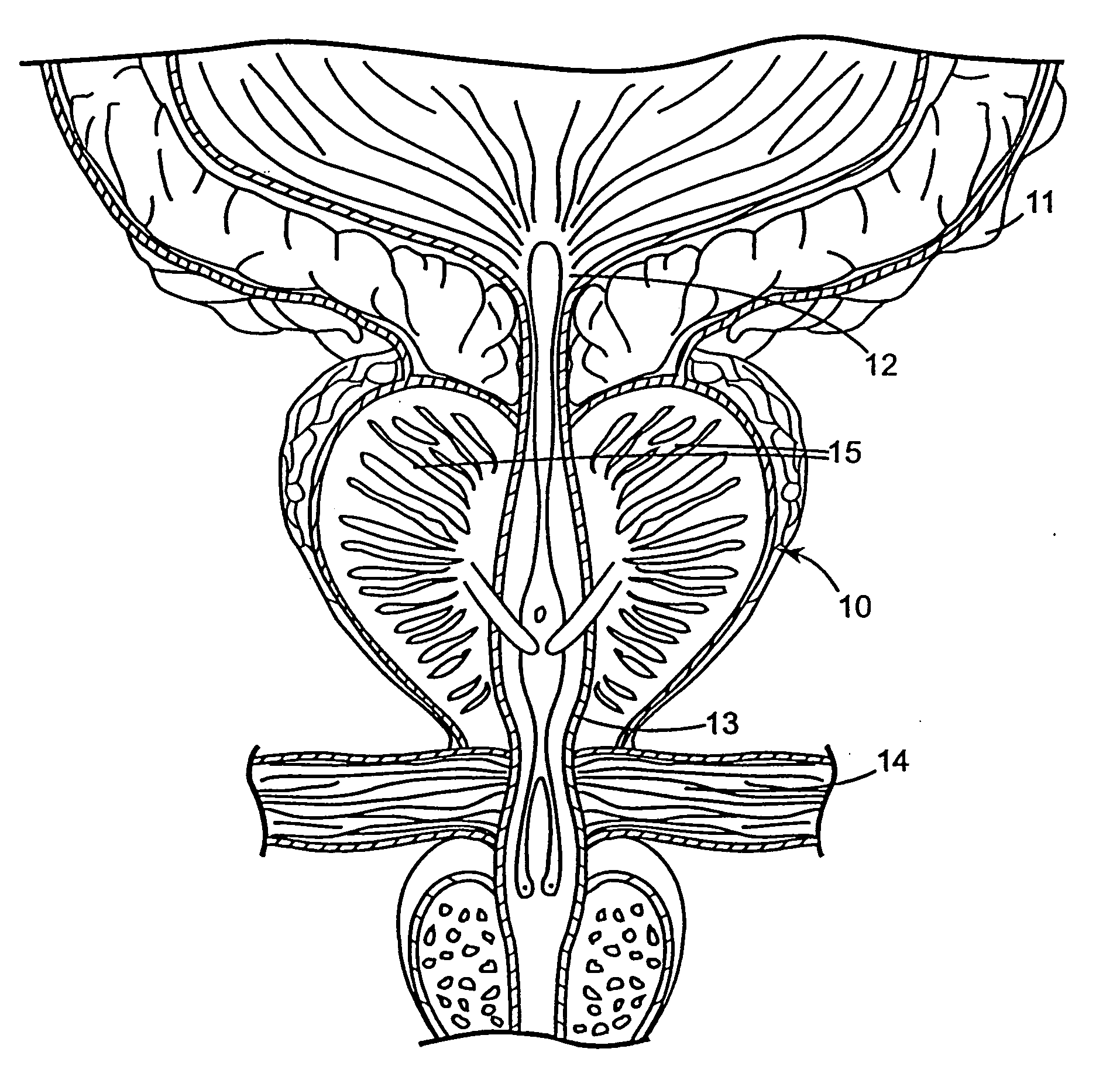
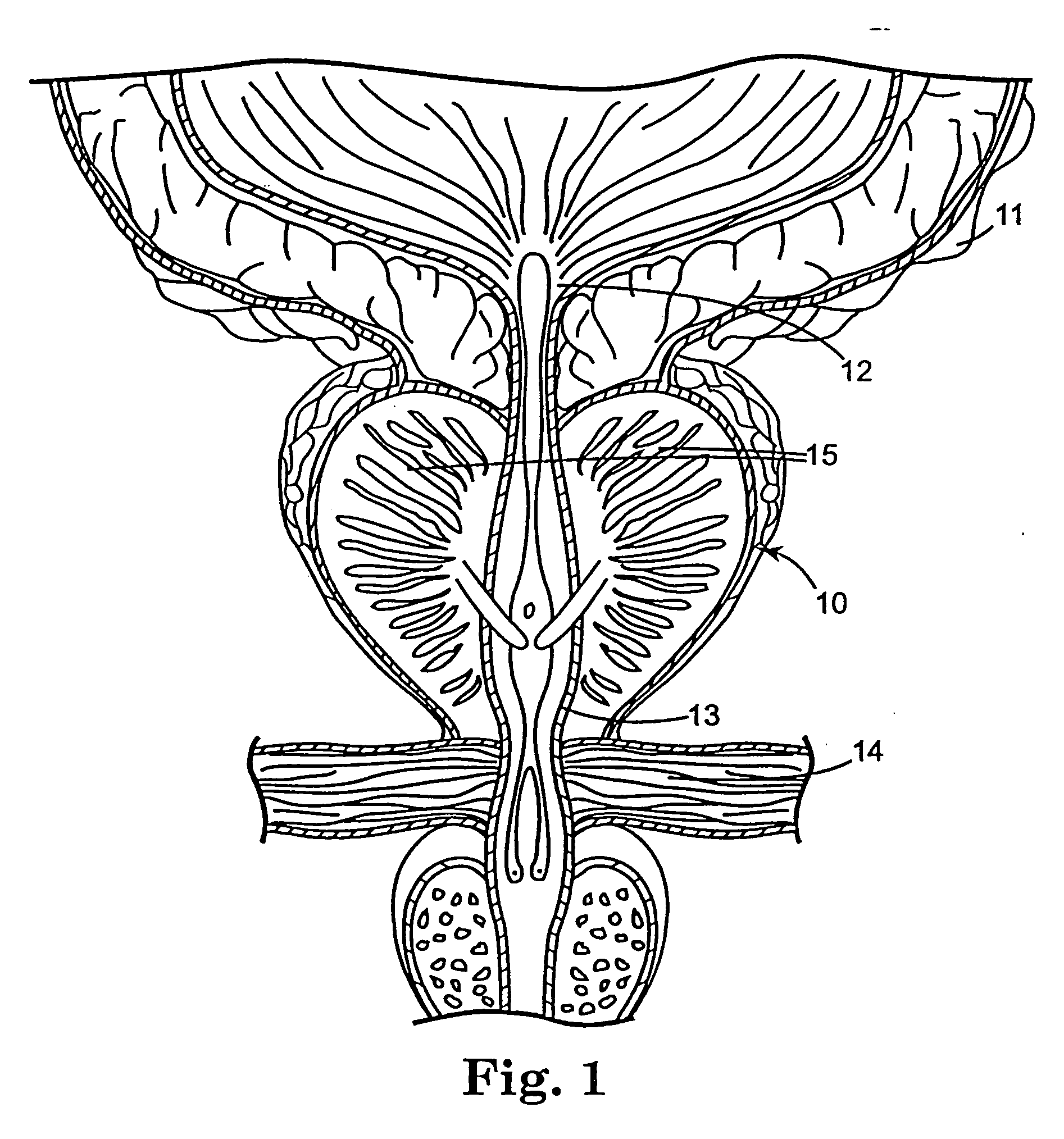
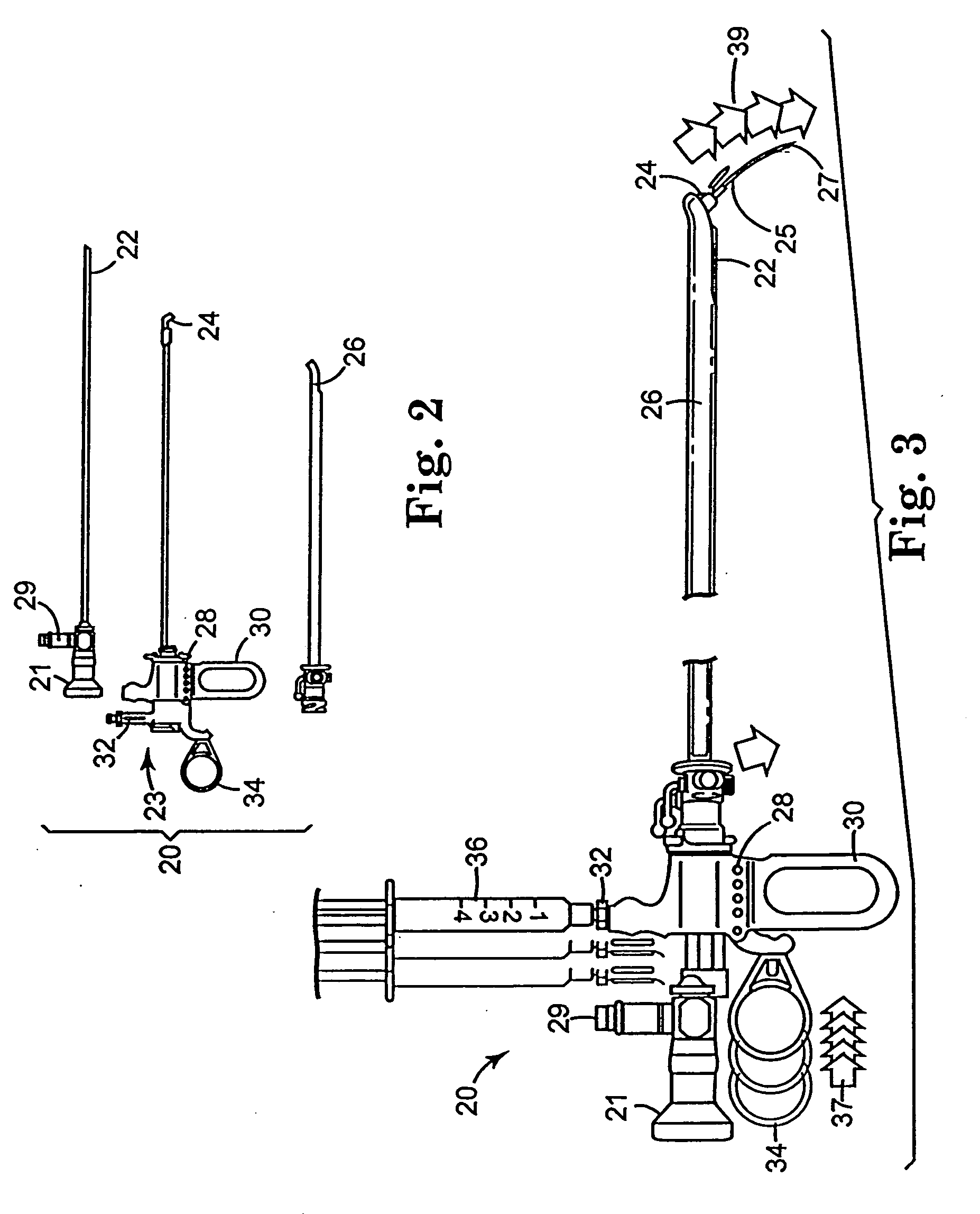
Regimen for treating prostate tissue and surgical kit for use in the regimen
a technology for prostate tissue and treatment regimens, applied in the field of treatment regimens for diseased prostate tissue and surgical kits, can solve the problems of difficult urination, frequent urination, and significant health risk of prostate disease for males, and achieve the effects of reducing prostate size, prostate size, and prostate siz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0122] Human patients exhibiting symptoms of BPH were treated by transurethral ethanol ablation of prostate tissue, with coadministration of the antiandrogen bicalutamide. The treatments for selected patients are summarized in Table 1. Measurements of each patient's prostate size were obtained prior to ethanol ablation by MRI. Flowmetry measurements were also taken. Maximum flow rate (Qmax) and median flow rate (Qmed) are given in Table 2.
[0123] Each patient was then administered ethanol ablation therapy by transurethral injection directly into prostate tissue, as summarized in Table 1. Total amount of ethanol injected ranged from about 22% to about 38% relative to the volume of the patient's prostate. The number of injection sites ranged from 3 to 11; the average number of injection sites was 5.8. Following ethanol ablation therapy, each patient was administered bicalutamide, 150 mg orally each day for sixty days, beginning on the day of treatment.
TABLE 1Summary of ethanol ablat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com