Methods and Compositions for the Treatment of Rhinovirus Infections with Carrageenan
a technology of carrageenan and rhinovirus, applied in the field of immunology and antiviral agents, can solve problems such as the development of vaccines, and achieve the effects of preventing or reducing the risk of infection, and being easy to incorporate into the tissue material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of Different Types of Carageenans Against Human Rhinovirus Type 2 and Type 14
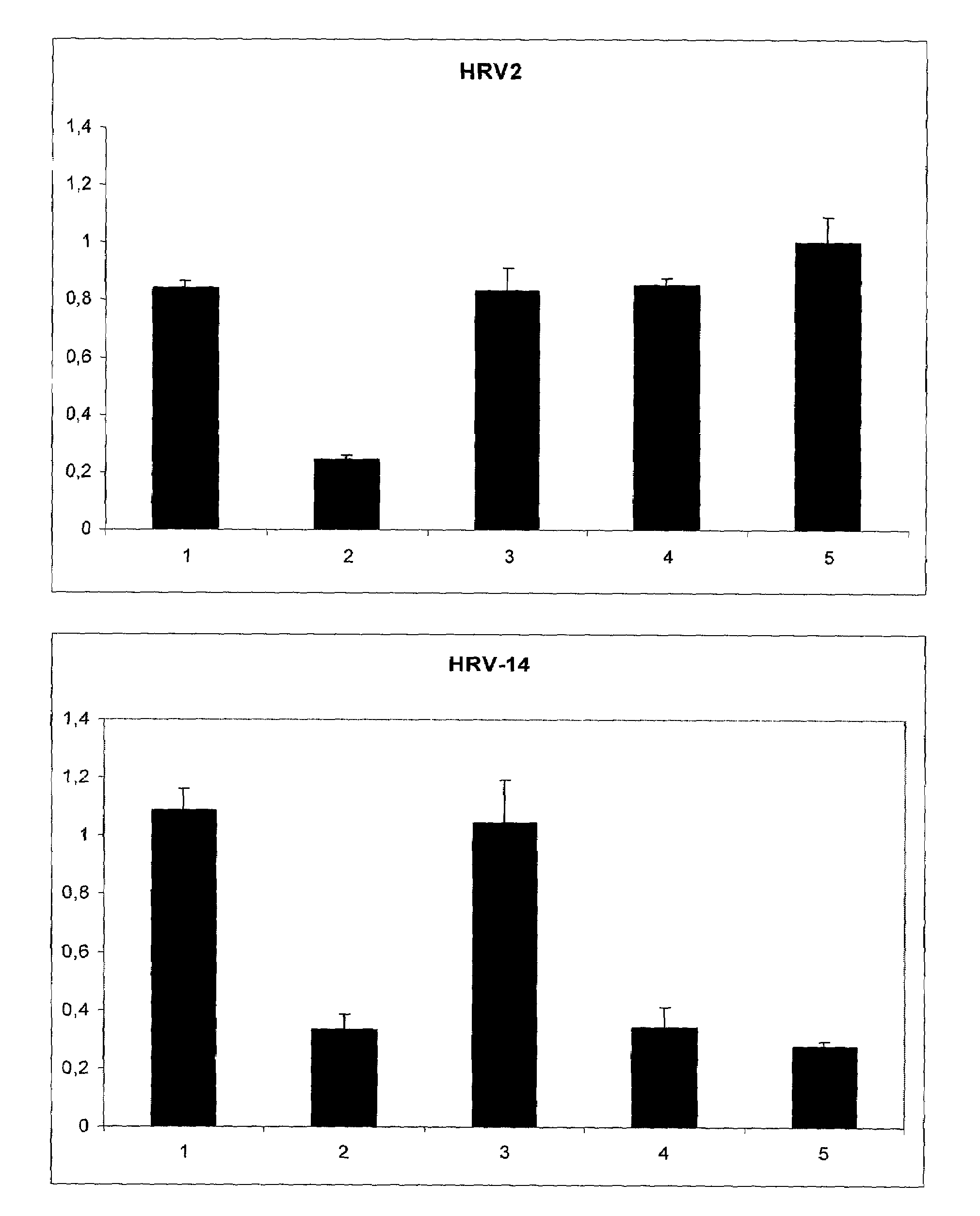
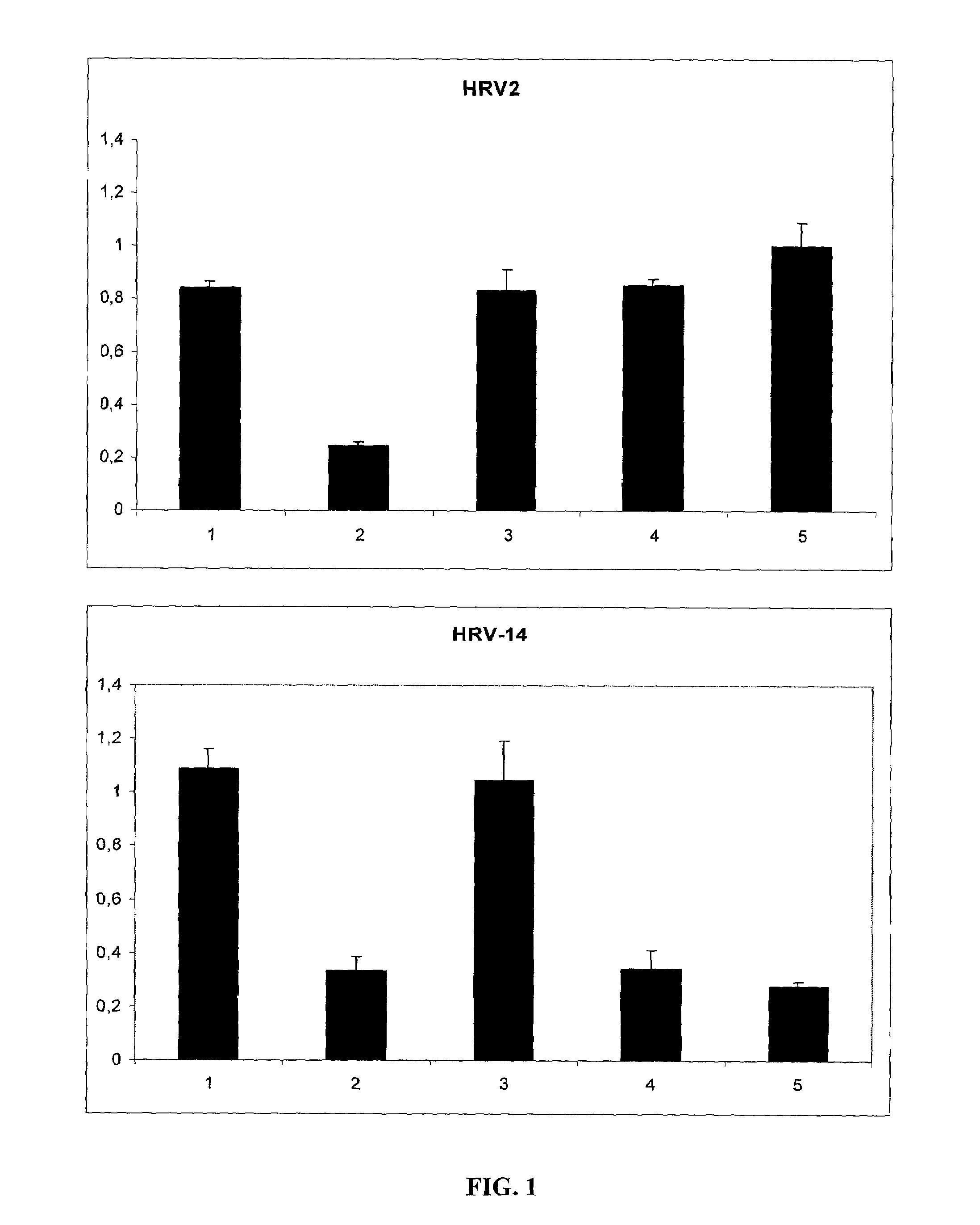
[0030]Subconfluent HeLa cells were incubated with a virus suspension that was preincubated for 5 min with 125 μg / ml of the indicated polymers. 48 hours later the viability of the cells was determined with TOX2 XTT assay (Sigma). (FIG. 1). Error bars indicate the standard deviation between six independent wells. The most effective polymer was iota-carrageenan that was effective against both types of rhinovirus while, lambda-carrageenan and kappa-carrageenan showed effectivity for HRV-2 but not for HRV-14.
example 2
[0031]The polymers indicated in Table 1 were tested in a HRV-2 and HRV-14 induced cell death inhibition assay (XTT-assay) at a concentration of 100 μg / ml: iota Carageenan showed protection against both types of Rhinovirus. Kappa Carageenan and lambda carrageenan, were active against HRV-2 but not against HRV-14. The polymers Chitosan, Carboxymethylcellulose and Carboxymethylchitosan did not show an inhibitory effect.
example 3
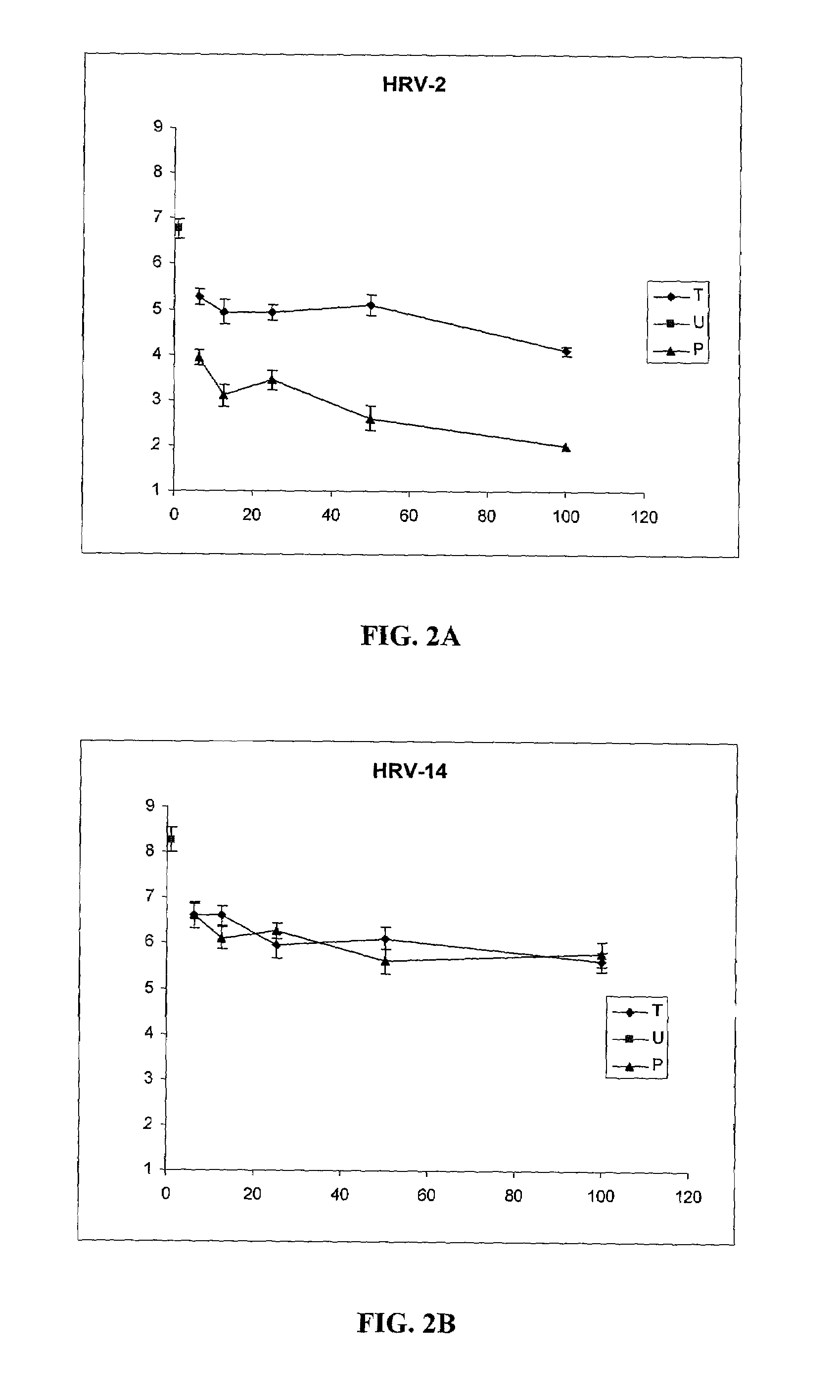
[0032]Iota-Carageenan is effective against HRV-2 and HRV-14 in a treatment and a prophylaxis viral replication model. A significant reduction of peak viral titer was observed at concentrations equal and higher than 6.25 μg / ml. However, iota carageenan was most effective in the prophylaxis model against HRV-2 in which a reduction of more than 99.9% in the peak viral titer was observed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com