Methods for increasing the production of ethanol from microbial fermentation
a technology of microbial fermentation and ethanol, which is applied in the field of methods for increasing the production of ethanol from microbial fermentation, can solve the problems of low productivity, unstable culture, and low productivity, and achieve the effect of allowing culture growth and good culture stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
An Exemplary Method of the Present Invention
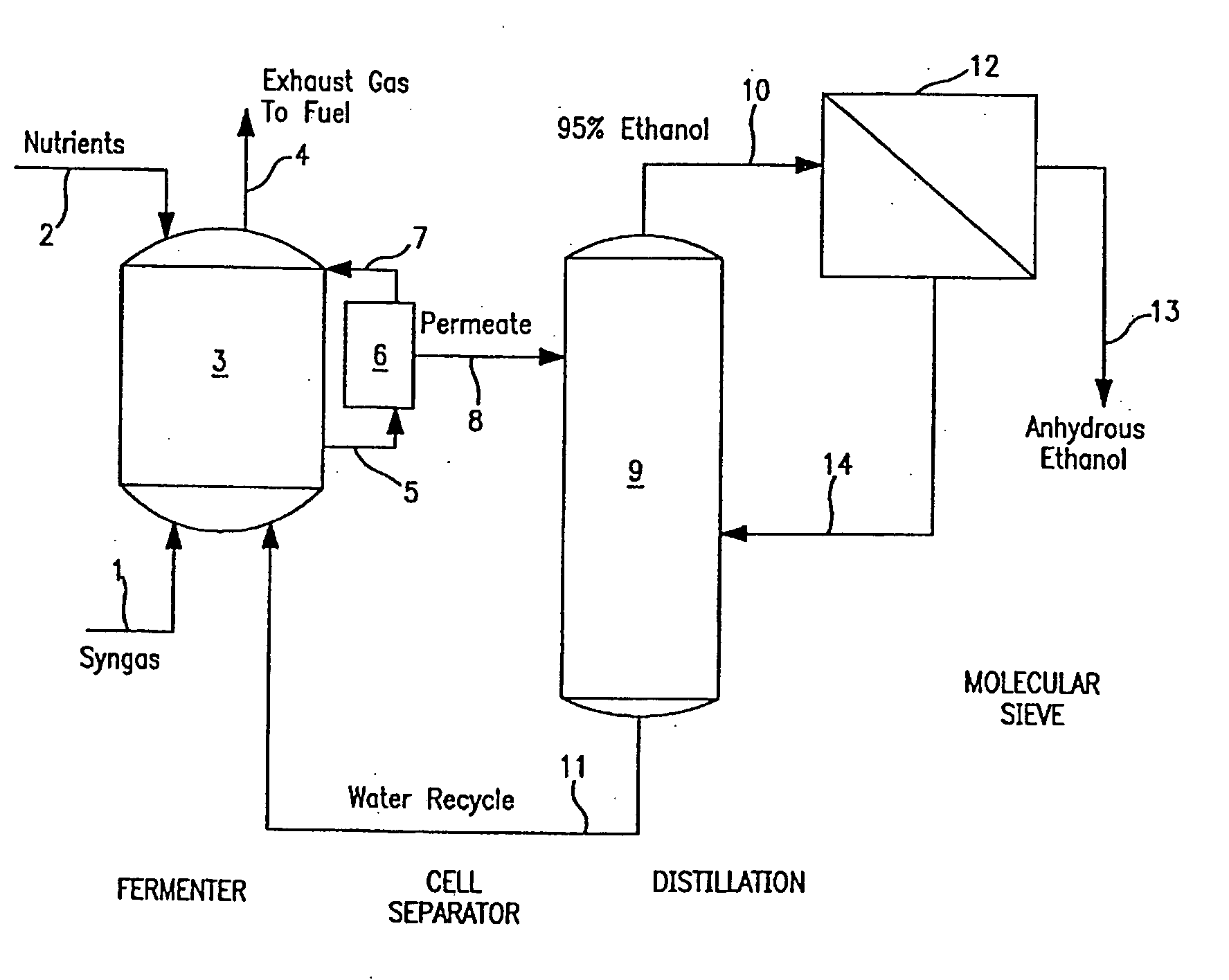
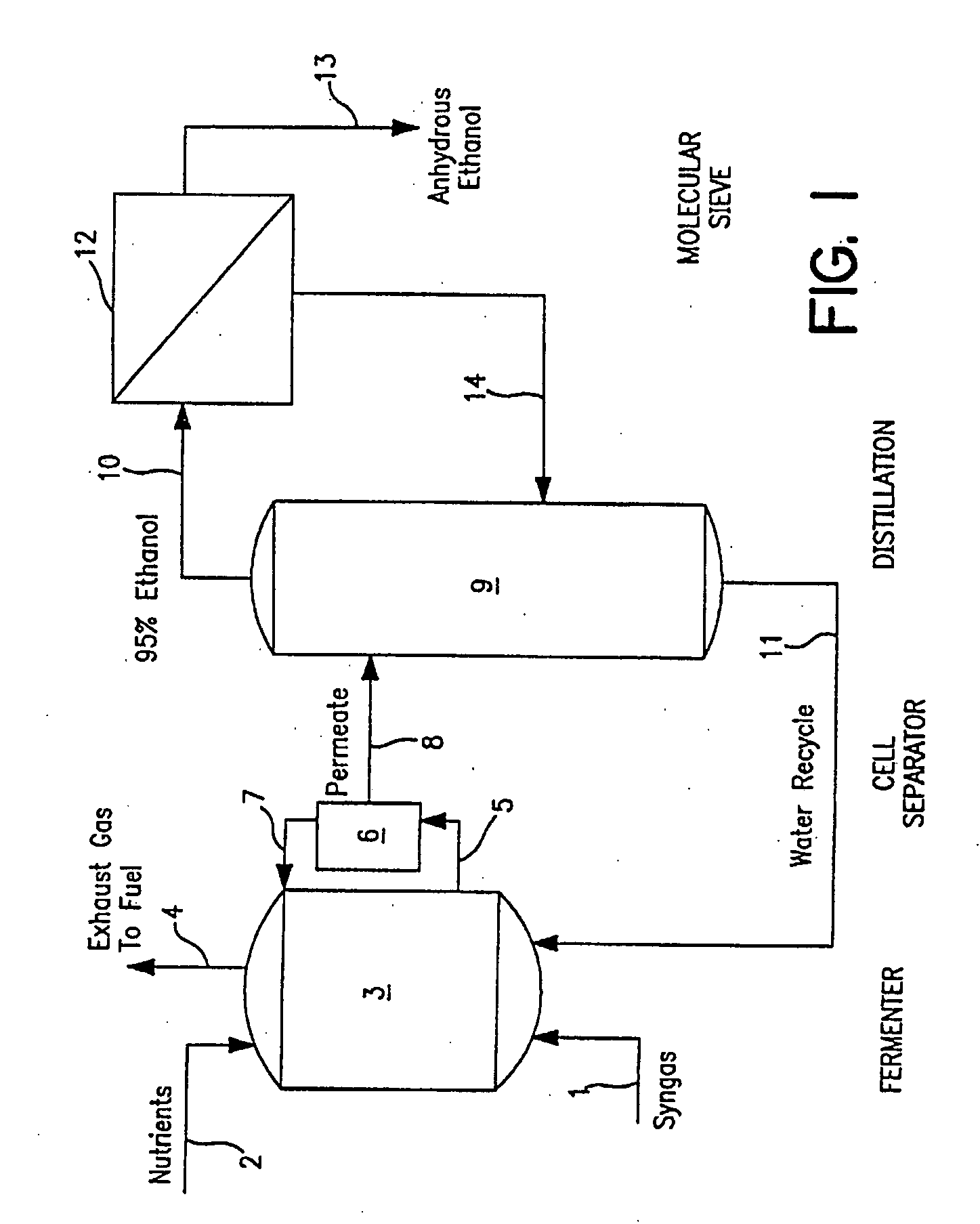
[0102]A synthesis or waste gas containing CO and / or CO2 / H2 is continuously introduced into a stirred tank bioreactor containing a strain of C. ljungdahlii, along with a conventional liquid medium containing vitamins, trace metals and salts. One desirable nutrient medium is reported in Table 1 below.
[0103]During method start-up using a culture inoculum of 10% or less the reactor is operated with a batch liquid phase, where the liquid medium is not fed continuously to the reactor. The liquid phase in the reactor thus consists of a batch of nutrient medium with a nominal concentration of limiting nutrient, either calcium pantothenate or cobalt. Alternatively, a rich medium containing yeast extract, trypticase or other complex nutrients can also be employed.
[0104]Ideally, the gas phase at start-up is CO2-free and contains excess H2. The gas rate and agitation rate are kept at low levels (less than 500 rpm in a New Brunswick Scientific Bioflo® ...
example 2
Sample Analysis Via Gas Chromatography
[0110]To achieve and / or maintain proper productivity and ratio, a sample of the fermentation broth in the fermentation bioreactor must be periodically sampled. A sample greater than 1.5 mL of culture is taken from the culture in the bioreactor. The sample is placed in a microcentrifuge tube and the tube is placed in a Fisher Scientific Micro 14 centrifuge with necessary ballast for balancing. The sample is subjected to 8000 rpm for 1.5 minutes. A 0.500 mL sample of supernatant is placed into a 1.5 mL vial designed for use in a gas chromatograph autosampler. A 0.500 mL sample of an internal standard solution containing 5 g / L of n-propanol and 5% v / v, 85% phosphoric acid in deionized water. The phosphoric acid assures the all acetate is converted to acetic acid and is detected by gas chromatography.
[0111]One μl of the prepared sample is then injected by autosampler into a Hewlett-Packard 5890 Series II Gas Chromatograph equipped with a 007 FFA Qua...
example 3
Acid Production in a Laboratory CSTR with Cell Recycle
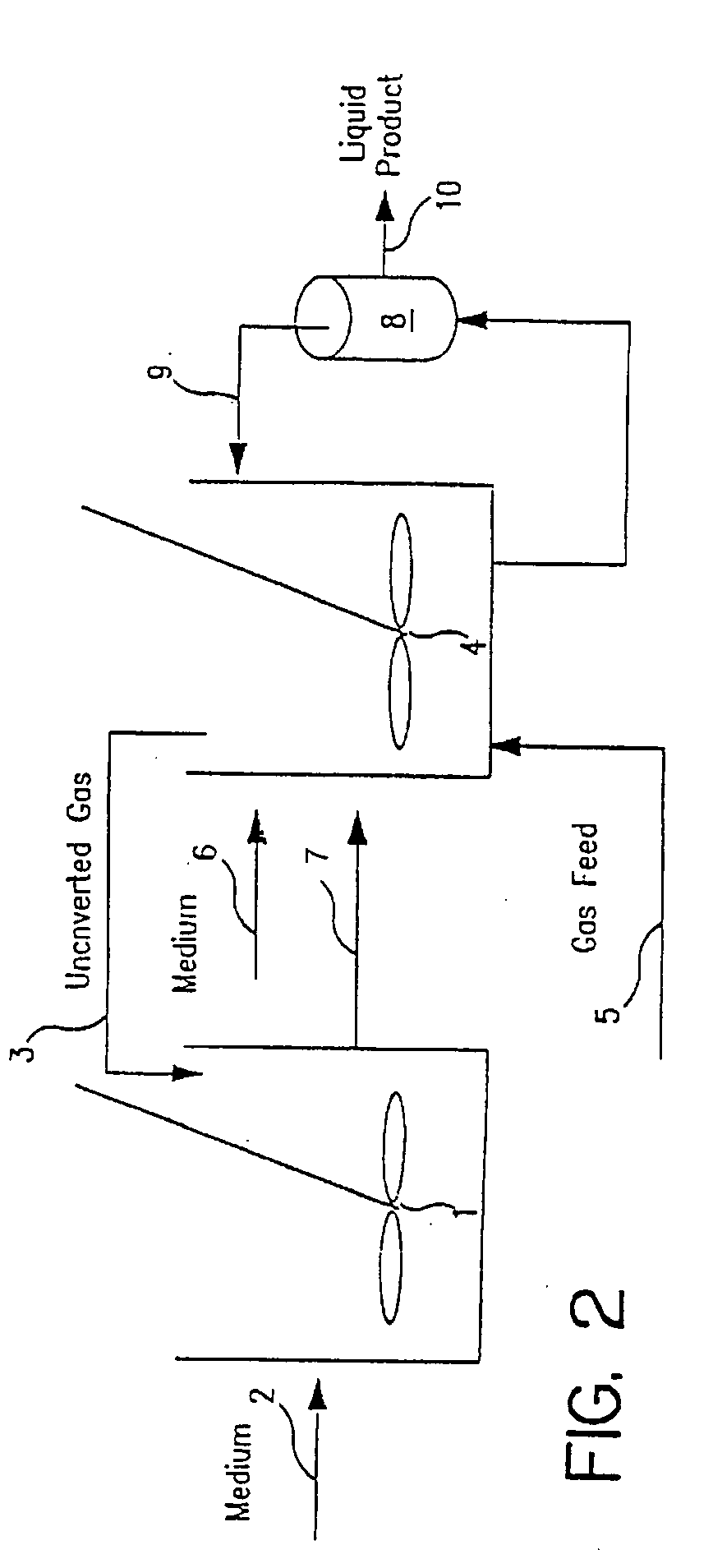
[0112]A New Brunswick Scientific Bioflo® laboratory fermentation bioreactor was operated with cell recycle using Clostridium ljungdahlii, strain ERI-2, ATCC 55380 for the production of acetic acid from CO, CO2 and H2. The gas feed contained 40% H2, 50% CO and 10% N2, and the gas retention time to the one-liter reactor was 7.7 to 8.6 minutes. Liquid medium containing vitamins, salts and trace elements was fed at a liquid retention time of 2.6 to 2.9 hours. The pH was 5.1 to 5.2, the agitation rate was 1000 rpm and the cell retention time was about 40 hours. Under these conditions of mass transfer limitation (and not nutrient limitation), the CO conversion was 94 to 98% and the H2 conversion was 80 to 97%. The cell concentration was 4 to 8 g / l, and acetate was produced at 10 to 13 g / L. No ethanol was produced. Although the reactor was operated under mass transfer limitation (limited by the ability to transfer gas to the culture) an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com