Induction of immunological tolerance
a technology of immunological tolerance and immunological response, applied in the field of immunological tolerance induction, can solve the problems of increasing the need for organs for transplantation, increasing the need for organs, and increasing the need for patients to be able to tolerate the treatment. , to achieve the effect of preventing the widespread use of immunological response, preventing the need for organ transplantation, and solving the shortage of organs for transplantation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Implantation of Mouse Insulinoma Cells
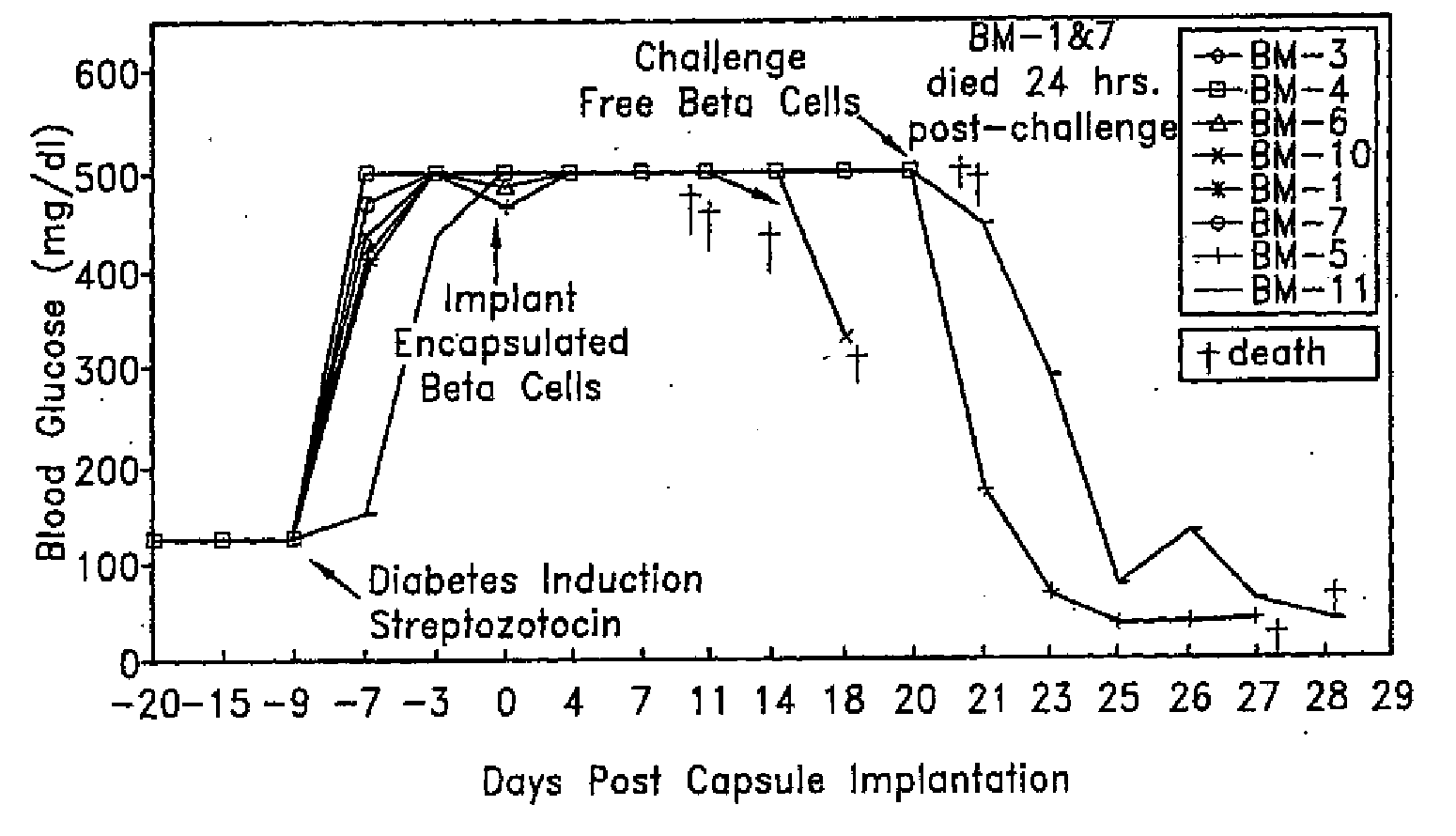
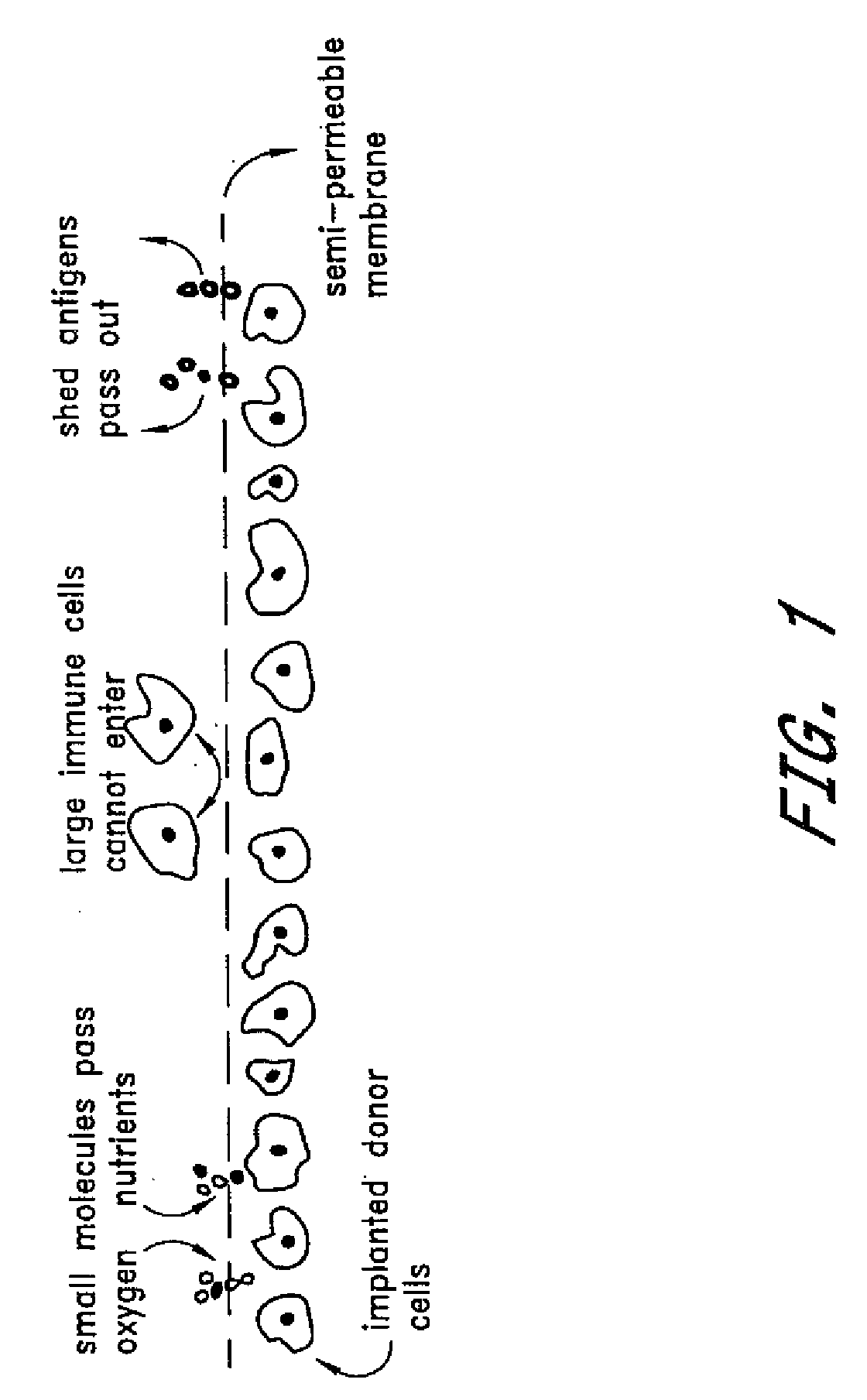
[0066]The NTT insulin-producing mouse tumor cell line was encapsulated with PEG conformal coatings of a single, configuration, 11% PEG 4,000 kDa molecular weight (See U.S. Pat. No. 5,529,914), which corresponds to a molecular weight cutoff of between about 10 kDa and about 70 kDa. The encapsulated cells were implanted beneath the kidney capsule at two different doses into C57B6 mice of a different allograft haplotype in which diabetes had been induced by intravenous injection (tail vein) of 167 mg / kg body weight of streptozotocin (Upjohn, Kalamazoo, Mich.). Induction of diabetes by streptozotocin injection is a well known procedure which destroys pancreatic insulin-producing β cells.
[0067]Tolerizing doses of encapsulated insulinoma cells were 50 or 100 cell aggregates, each containing about 1,000 cells. Encapsulated cells were implanted beneath the kidney capsule using standard surgical procedures. Curative implants of unencapsulated insulinoma ...
example 2
Use of Encapsulated Islets for Induction of Allograft Tolerance in Rats
[0071]Rat pancreatic islet cells are isolated by a standard collagenase digestion method (Ricordi, Diabetes 37:413-410, 1988) and cultured for three days prior to PEG encapsulation. Donor islets are derived from the Wistar Furth (WF) strain having MHC haplotype RT1-U. Recipients are of the Lewis strain having MHC haplotype RT1-1. Transplants across this strain combination are normally rejected within three weeks. Islet transplant mass is dosed on the basis of a standard 150 μm diameter rat islet; an Islet Equivalent (Ieq). Islets are quantified and tested for sterility and mycoplasma prior to encapsulation and implantation.
[0072]Islet cells are conformally coated with 11% PEG 4,000 kDa molecular weight by the method described in U.S. Pat. No. 5,529,914. As a negative control, acellular cross-linked dextran beads are encapsulated in a similar manner. Diabetes is induced in fasted Lewis rats by intravenous injectio...
example 3
Use of Encapsulated Islets for Induction of Allograft Tolerance in Humans
[0079]Human islets are isolated from cadavers and 1,500 islets / kg body weight are PEG-encapsulated and implanted under the kidney capsule in a diabetic patient After two months, a curative dose of 15,000 unencapsulated islets / kg body weight are injected intraportally. Insulin administration is continued during the course of the protocol up to administration of the curative dose. Blood glucose levels are constantly monitored and are within the normal range.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com