Stabilized exendin-4 compounds
a technology of exendin-4 and compound, applied in the direction of peptide/protein ingredients, peptide sources, metabolism disorders, etc., can solve the problem of glp-1's short half-life, and achieve the effects of stable exendin-4 compound and related composition, improved consistency between lots, and more reliable activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Stabilization of Exendin-4 and Related Molecules by Structural Isomerization and / or Oxidation
[0222]Pharmaceutical compositions of Exendin-4(1-39) or a variant, analogue, or derivative thereof or aqueous solutions of Exendin-4(1-39) or a variant, analogue, or derivative thereof can be stabilized by oxidation or functional isomerization at various points in the Exendin-4(1-39) sequence.
[0223]Storage of the Exendin-4(1-39) or a variant, analogue, or derivative thereof in an aqueous solution at a temperature of between about 0° C. and about 50° C., more particularly between about 4° C. and room temperature can induce a structural rearrangement at the 28-L-Asparaginyl residue of the Exendin-4(1-39) peptide. Suitable aqueous solutions are not particularly limited and may include aqueous pharmaceutical compositions which may have one or more additional additives to facilitate administration to a patient or to stabilize or solublize the Exendin-4(1-39) or a variant, analogue, or derivative ...
example 2
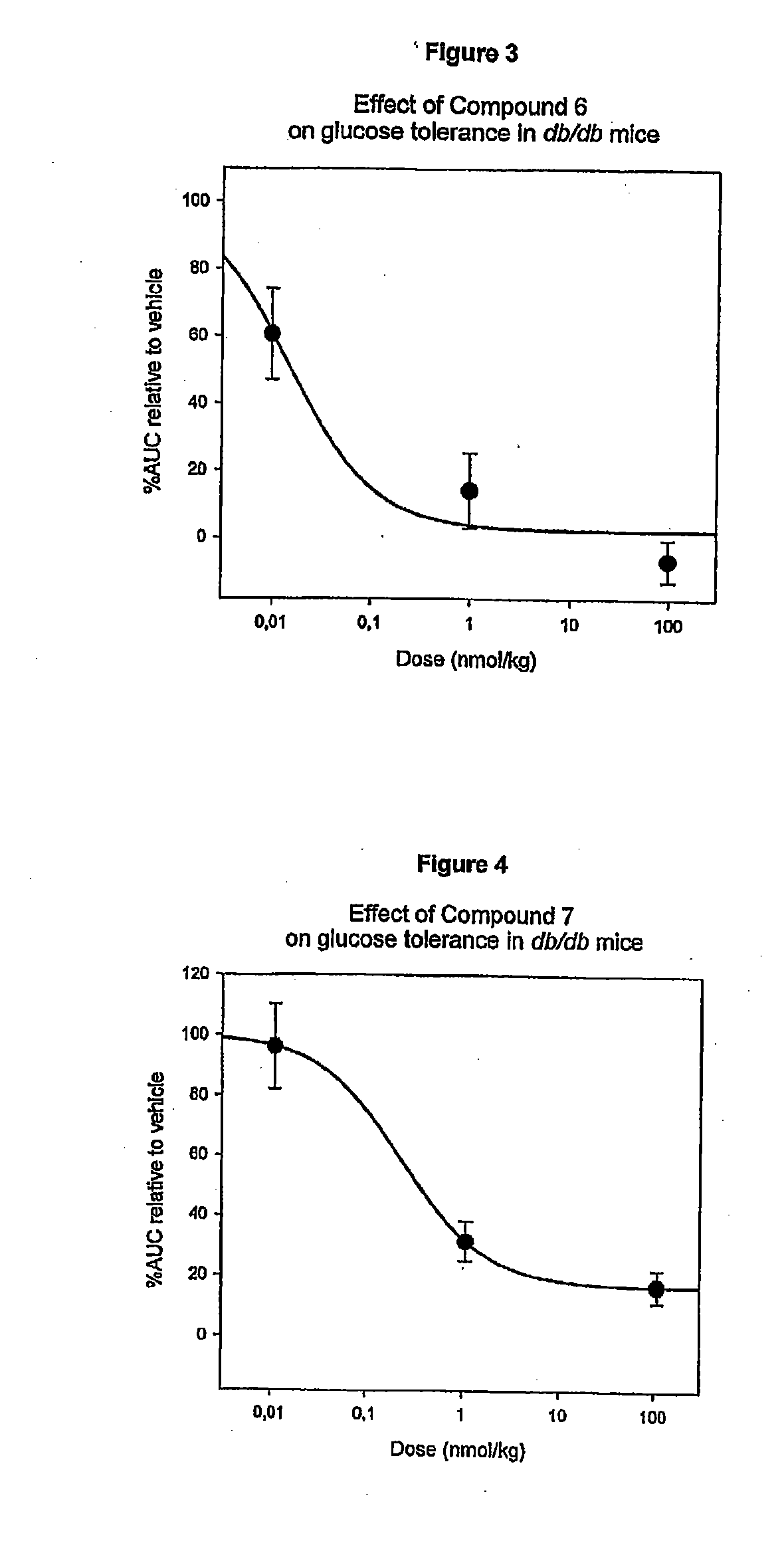
Synthesis of Compound 5, 6 and 14 (Stabilized Derivatives of Compound 1)
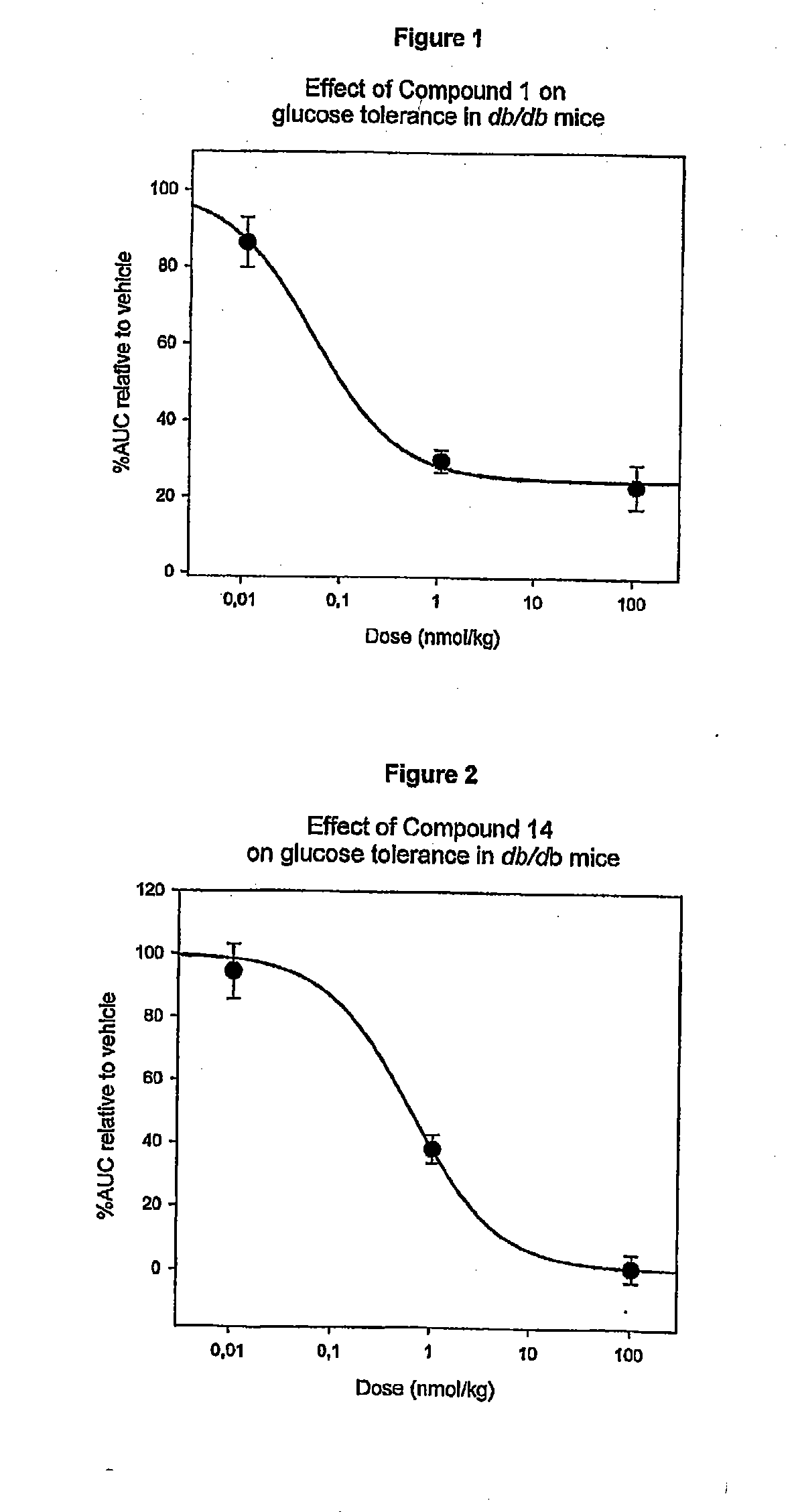
[0229]Compound 1 (des Pro36 Exendin-4 (1-39)-K6; SEQ ID NO:1) has the structure shown in FIG. 1 and it was made using the Merrifield technique. See the PCT / DK00 / 00393 application, for example, for more information.
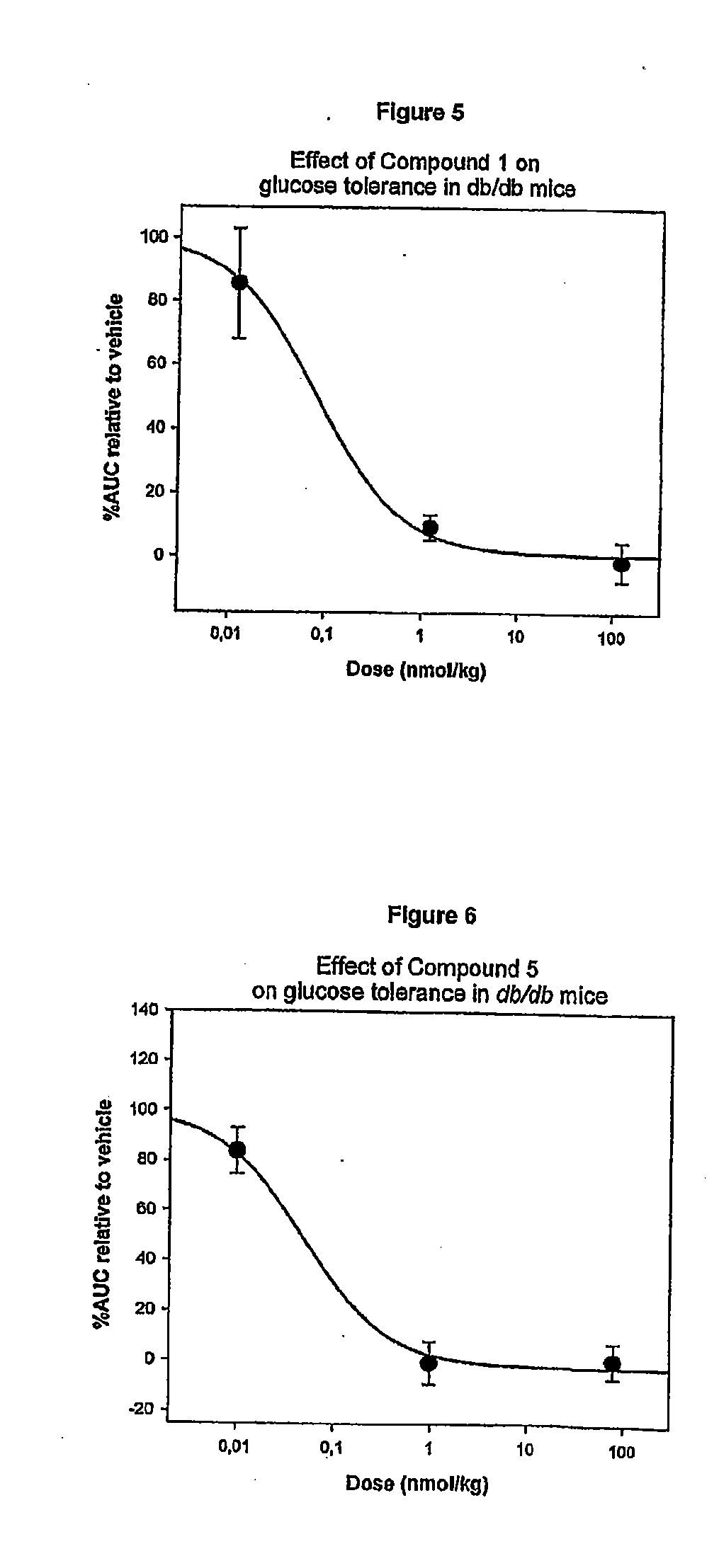
[0230]About 458 mg of Compound 1 was dissolved in 100 mM NH4HCO3 pH 7.9 to a concentration of 10 mg / mL. The solution was incubated at 40° C. for 6 days to yield approx. 20% Compound 5, 10% Compound 14 and 50% Compound 6.
[0231]The stabilized product Compound 5 and Compound 6 can be purified by preparative RP-HPLC isocratic elution or using a gradient, respectively. Identification is accomplished by relative retention time in combination with Amino acid Sequencing and LC-MS (ESI+ / TOF)
example 3
Synthesis of a Compound 7 (Stabilized Derivative of Compound 1)
[0232]About 424 mg of Compound 1 was dissolved in 100 mM NH4HCO3 pH 7.9 to a concentration of 10 mg / mL. The solution was incubated at 40° C. for 5 days to yield approx. 20% Compound 5, 60% Compound 6. Compound 6 was purified by preparative RP-HPLC. Approximately 100 mg Compound 6 was obtained and lyophilised. About 100 mg Compound 6 was reconstituted in 100 mM NaH2PO4 buffer and adjusted to pH 5.3 with NaOH to a concentration of 5 mg / mL. The solution was incubated at 40° C. for 5 days to yield approximately 40% Compound 7.
[0233]The stabilized product Compound 7 can be purified by preparative RP-HPLC gradient elution. Identification is accomplished by relative retention time in combination with LC-MS (ESI+ / TOF).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| Room temperature | aaaaa | aaaaa |
| yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com