Use of PI3K M-TOR and Akt Inhibitors to Induce FOXP3 Expression and Generate Regulatory T Cells
a technology of foxp3 and m-tor, which is applied in the field of new methods for inducing foxp3 expression in t cells, can solve the problem of no demonstration of de novo foxp3 expression using such a techniqu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Withdrawal of TCR Signal Induces Foxp3 Expression in Newly Activated T Cells
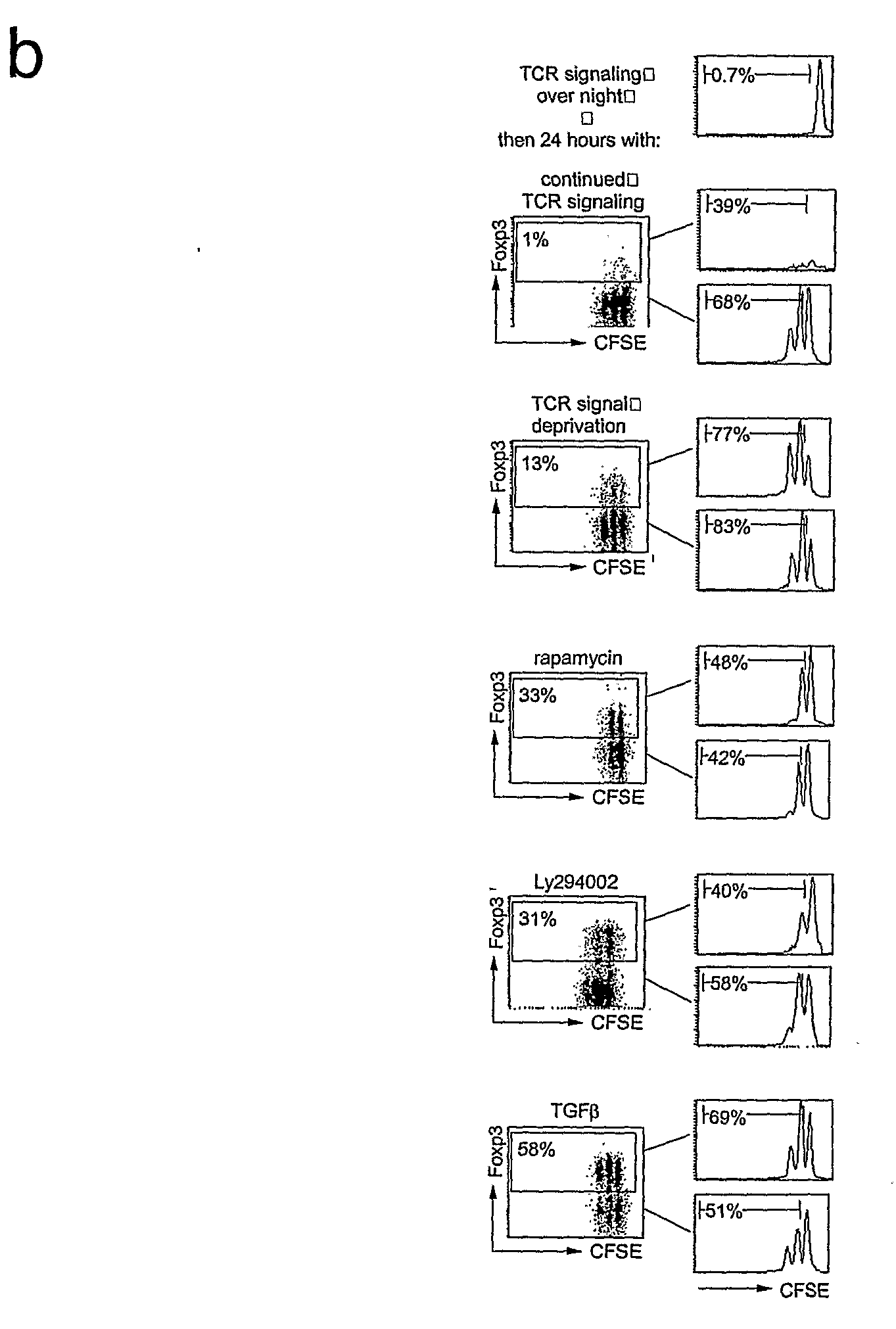
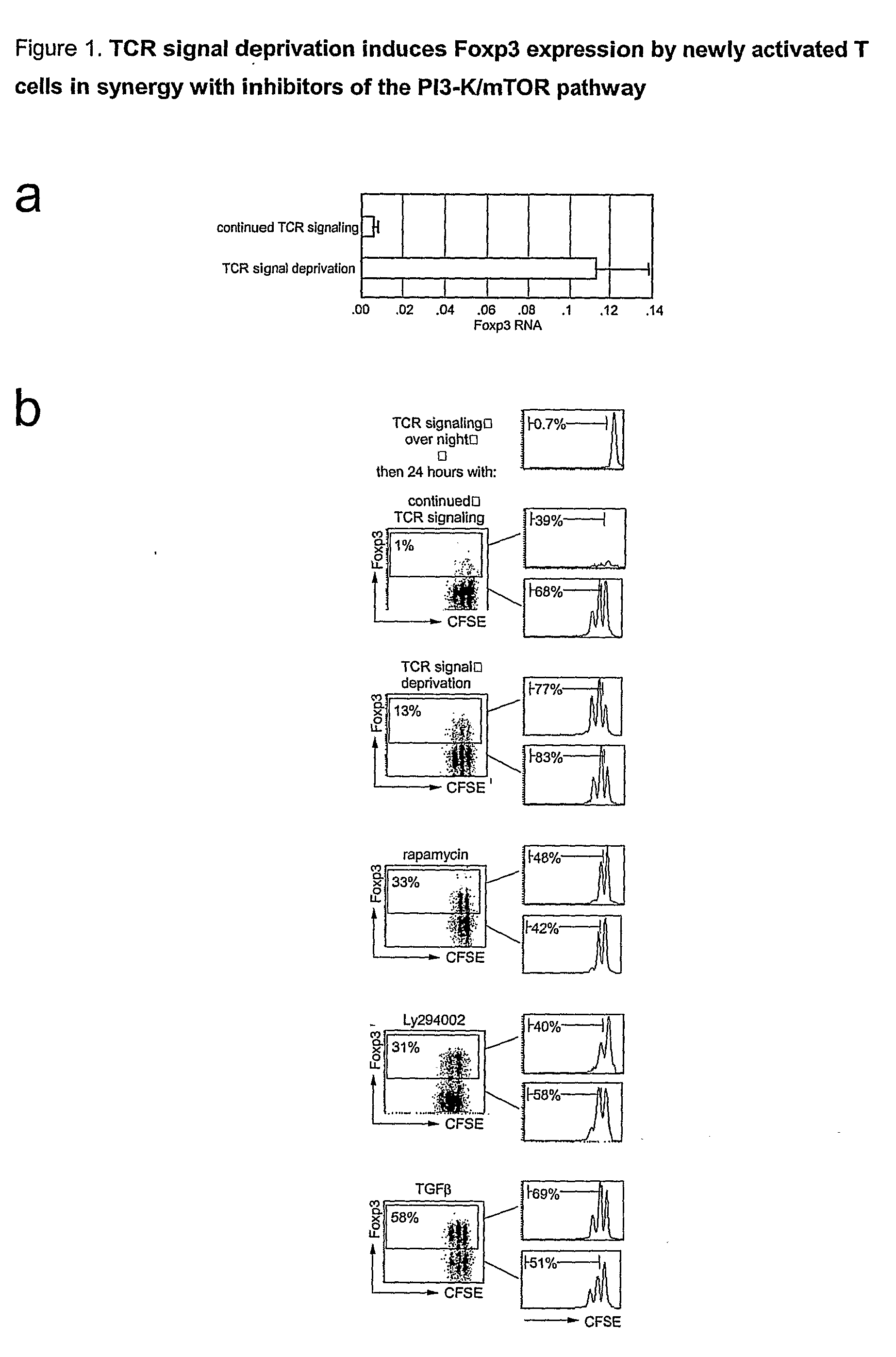
[0177]Naive CD4+ CD25− cells were stimulated with plate bound anti-TCR(H57) and anti-CD 28 for 18 hours and then transferred (in their original medium) to plates not coated with TCR antibodies. 48 hours later, real time RT-PCR showed elevated levels of Foxp3 RNA in cells deprived of TCR signals compared to controls exposed to continued TCR signaling (FIG. 1a). Intracellular staining showed the expression of Foxp3 protein in a sizeable fraction of newly activated CD4 T cells deprived of TCR signals (10.8±7.6% n=30) (FIG. 1b), but not in cells left in contact with TCR antibody (1.0±0.8% n=21).
example 2
Inhibition of the PI3K / mTOR Pathway Promotes Foxp3 Expression in Newly Activated T Cells
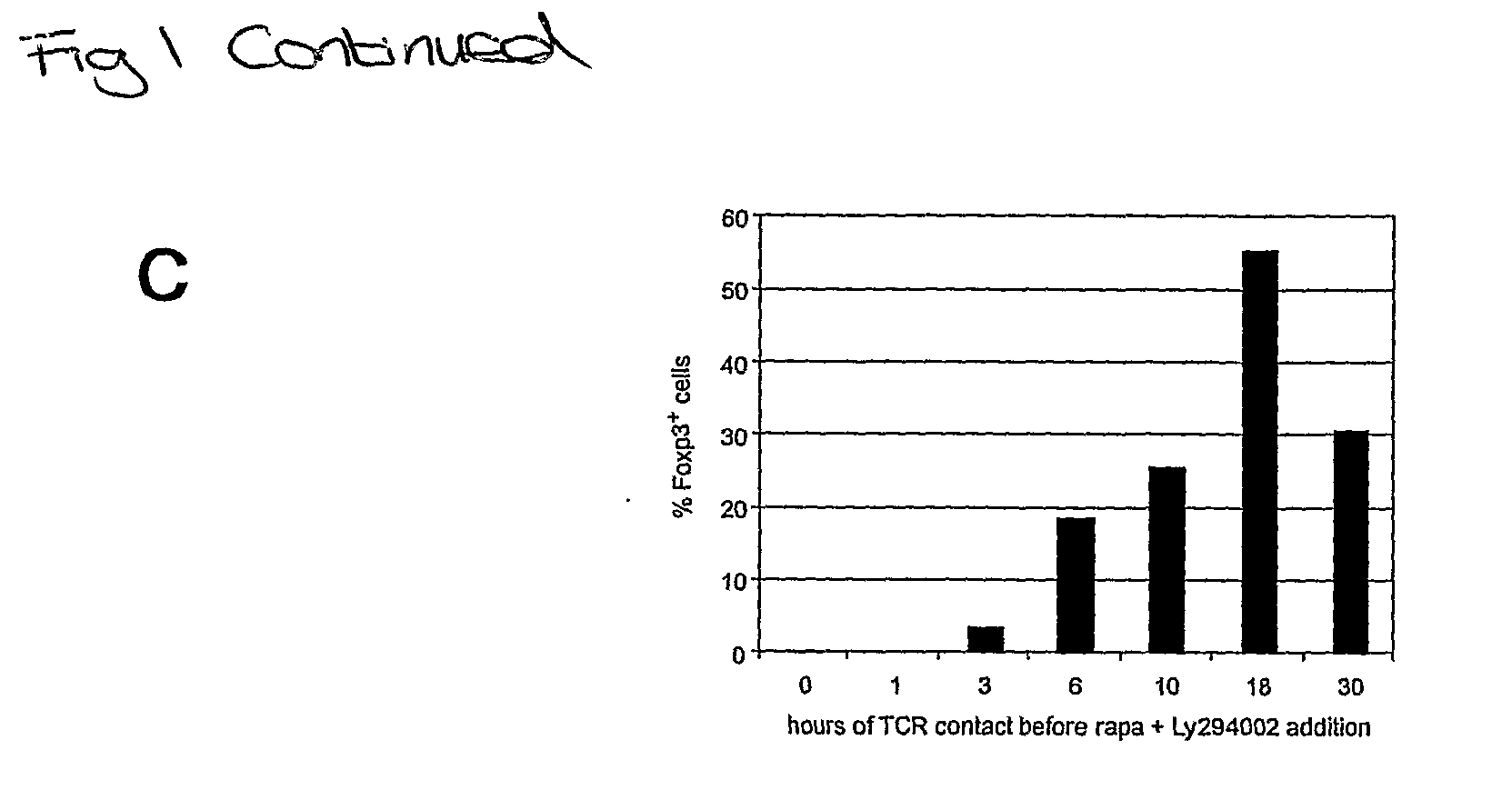
[0178]To identify relevant signaling pathways for the induction of Foxp3 we screened activators and inhibitors of signal transduction pathways. These included inhibitors of calcineurin, which block the nuclear translocation of NFAT (cyclosporin A and FK-506), activators and inhibitors of relevant MAPKs (mitogen activated kinases, including JNK / SAPK / p38 and upstream kinases), a wide range of inhibitors and pseudosubstrates of PKC isoenzymes, GSK3 (glycogen synthase kinase-3), HIF-1□(hypoxia inducible factor), Notch (using the β-secretase inhibitor L-685458) and BMP (bone morphogenetic proteins, which are relatives of TGFβ. None of these delectably enhanced the expression of Foxp3 initiated by TCR signal deprivation. In contrast, inhibitors of PI3K and mTOR markedly potentiated Poxp3 induction. The immunosuppressive macrolide antibiotic rapamycin induced the expression of Foxp3 in 26.8±14.4% of cel...
example 3
TCR Signalling Controls mTOR Activity in Newly Activated CD4 T Cells
[0180]To monitor the activity of the PI3K / mTOR axis in response to TCR signaling, TCR signal withdrawal and treatment with rapamycin and Ly294002, we analysed the level of phosphorylated S6 ribosomal protein (pS6), the target of p70 S6 kinase, which is directly regulated by mTOR. Intracellular staining for pS6 showed at the single cell level that TCR signaling (plate bound H57, 300 ng / ml) combined with anti-CD28 induced high levels of S6 phosphorylation in the majority of naive CD4 T cells (FIG. 2a). Anti-CD28 alone had no effect (FIG. 2a, top panel). S6 phosphorylation persisted in the presence of continued TCR signaling, but was completely removed by rapamycin (25 nM) or Ly294002 (10 μg / ml) within one hour (FIG. 2b). pS6 declined more gradually when naive CD4 T cells that had been activated for 18 hours with plate bound H57 and anti CD28 were deprived of TCR signals (FIG. 2b). Hence, Foxp3 induction occurs in resp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com