Glycomacropeptide medical foods for nutritional management of phenylketonuria and other metabolic disorders
a technology of phenylketonuria and glycomacropeptides, applied in the field of medical foods, to achieve the effect of increasing dietary compliance and improving quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Supplemented Glycomacropeptide Diet in Murine Model of PKU
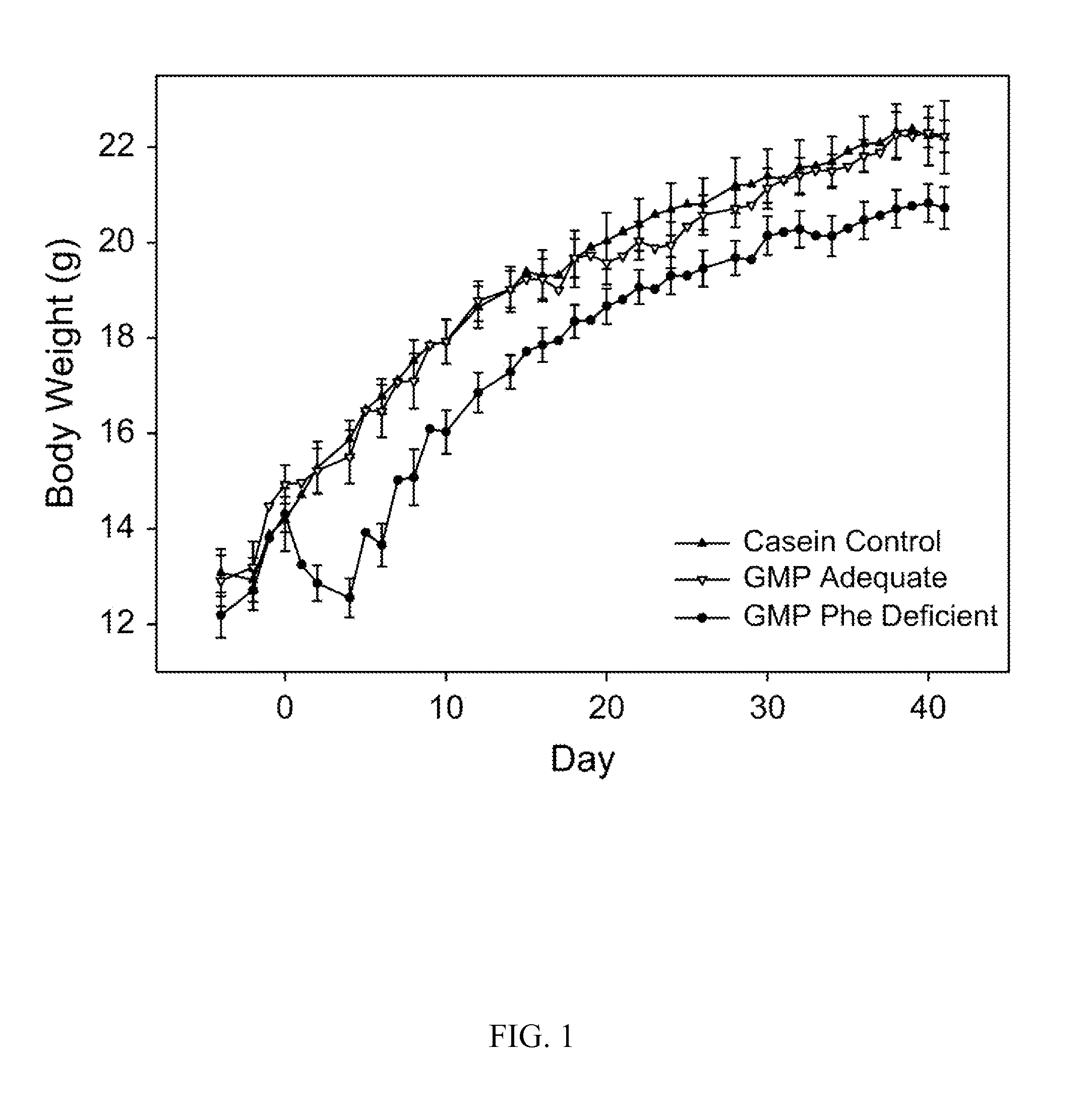
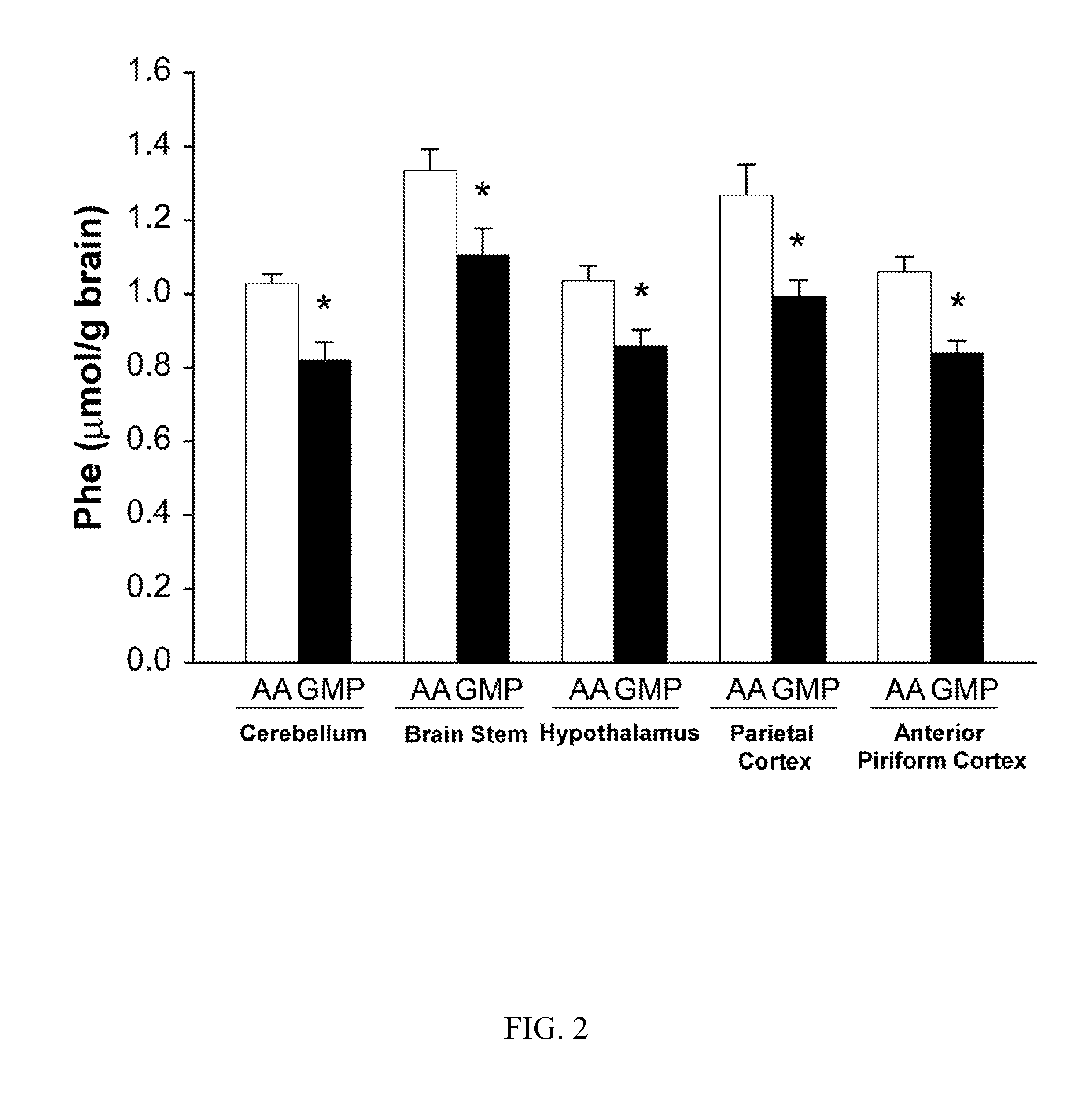
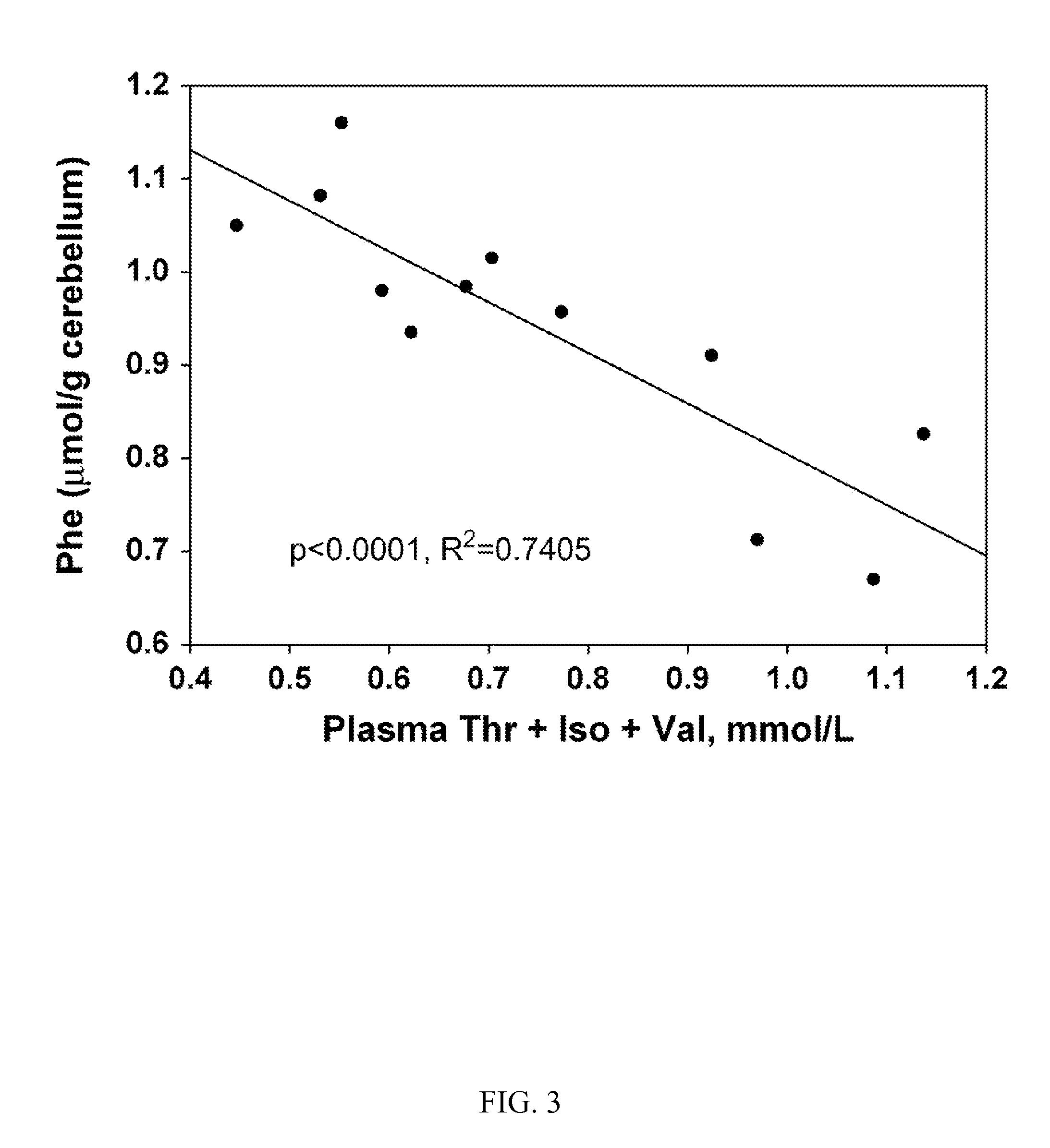
[0087]In this example, Applicants demonstrate that in a standard mouse model of PKU, a supplemented glycomacropeptide diet supports growth and reduces phenyalanine concentrations in both plasma and brain, as compared an amino acid diet. The murine model of PAH deficiency, the Pahenu2 mouse (PKU mouse) is a suitable model to study the nutritional management of PKU as it exhibits hyperphenylalaninemia and cognitive defects similar to humans with PKU. Moreover, parallel to the human low-Phe diet in which the majority of dietary protein is provided by amino acids, studies in the PKU mouse utilize an amino acid-based diet often free of Phe with provision of Phe in drinking water. Our objective was to assess how ingestion of diets containing GMP as the sole protein source support growth and impact the concentrations of amino acids, in particular Phe, in plasma and brain of wild-type (WT) and PKU mice. The results demonstated suitab...
example 2
Palatability of Foods Made with GMP and Supplemental AAs
[0125]In this Example, Applicants made a variety of palatable, low-phe foods and beverages with GMP and assessed their acceptability by conducting consumer sensory studies in individuals with PKU. Results demonstrate acceptability of products made with GMP.
[0126]Materials and Methods
[0127]Foods and Beverages.
[0128]Strawberry pudding, strawberry fruit leather, chocolate beverage, snack cracker and an orange sports beverage containing GMP were developed for this study in the Food Applications Laboratory at the Wisconsin Center for Dairy Research (CDR), University of Wisconsin-Madison (UW). The BioPURE-GMP (Davisco Foods International, Inc., LeSueur, Minn.) was used to formulate GMP products. The amino acid profile of BioPURE-GMP compared to casein is shown in FIG. 4.
[0129]A commercial amino acid-based chocolate beverage and low protein cracker were included in the taste testing to provide comparisons between GMP products and prod...
example 3
Case Study of Adult with PKU Following a Ten Week GNP Diet
[0140]This Example is a case report of a 29 year old male with PKU who used GMP-based foods as his sole protein source for a ten week period. The test subject reported that GMP-based foods tasted better than the standard amino acid formula, and his plasma levels of Phe were lower overall for the ten weeks that he consumed the GMP-based diet.
[0141]Approval was granted by the Health Sciences Institutional Review Board, University of Wisconsin-Madison to conduct an outpatient study in subjects with PKU to evaluate the safety and acceptability of dietary GMP. A 29-year-old male PKU subject with a genotype of R261Q and R408W was studied. The subject adhered to the low-Phe diet from birth through 12 years but was of diet during adolescence, which resulted in the development of spastic quadriparesis and a seizure disorder that was treated with standard anticonvulsant therapy. The subject completed a 15-week study comparing GMP with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com