Anti-garp protein and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
New Monoclonal Antibodies Directed Against Human GARP (Anti-hGARP Monoclonals)
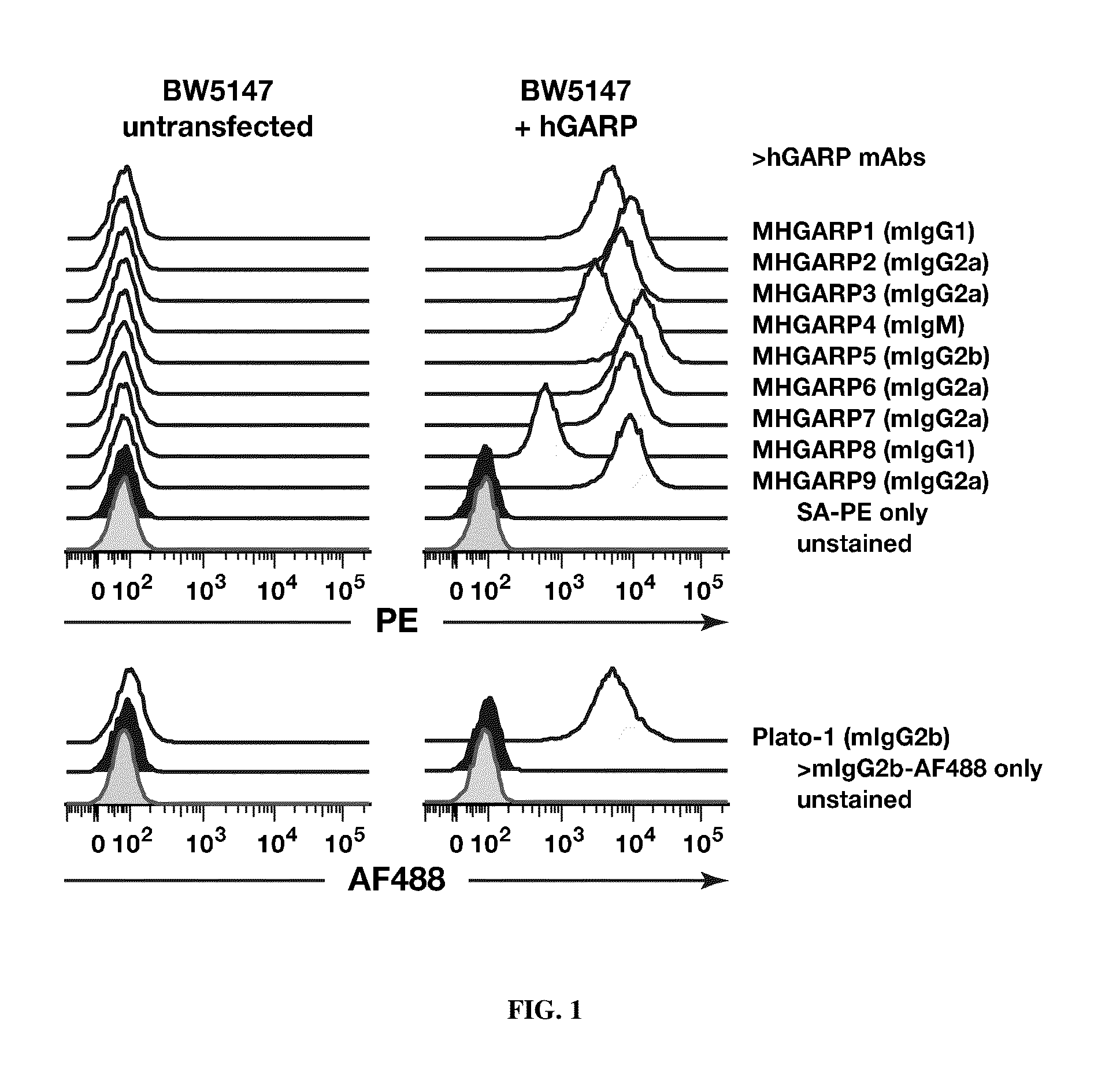
[0324]DBA / 2 or Balb / c mice were immunized with murine P1HTR cells transfected with human GARP. Sera from immunized mice were tested for the presence of anti-hGARP antibodies, by screening for binding to hGARP-expressing BW cells by FACS. Splenocytes from mice with high titers of anti-hGARP antibodies were fused to SP2 / neo cells. Hybridomas were selected in HAT medium and cloned under limiting dilution. Supernatants of + / −1600 hybridoma clones were screened by FACS for the presence of antibodies binding to hGARP-expressing BW cells. Thirty-eight clones producing anti-hGARP monoclonal antibodies were identified in this screening. Nine clones were selected and amplified for large scale-production and purification of nine new anti-hGARP monoclonals (MHGARP1 to 9).
[0325]As shown in FIG. 1, MHGARP1 to 9 bind to murine BW5147 cells transfected with hGARP, but not to untransfected cells. MHGARP1 to 9 also bind 293...
example 2
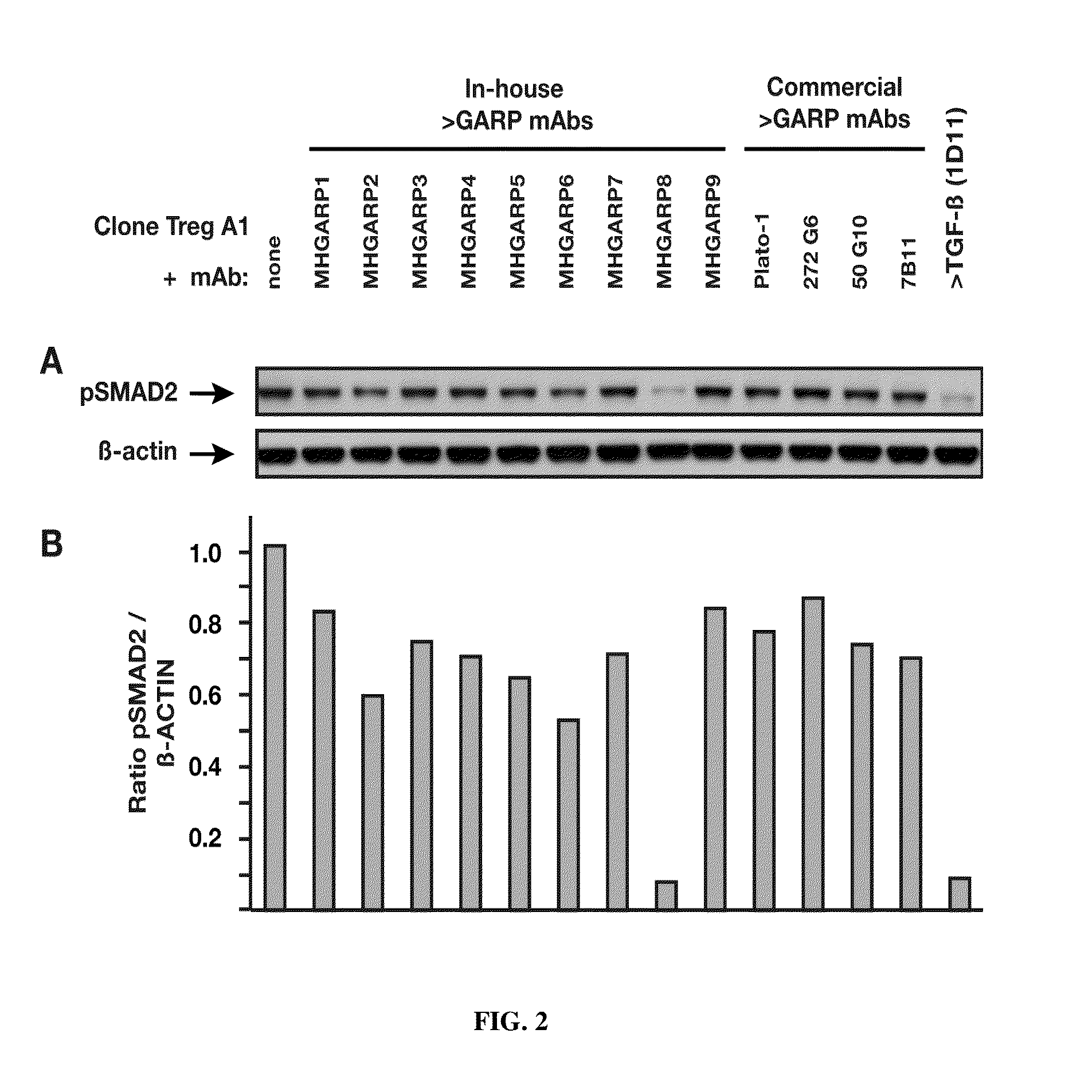
MHGARP8, but None of 12 Other Anti-hGARP Monoclonals, Inhibits Active TGF-β Production by Human Treg Cells
[0327]A human Treg clone (1E+06 cells / ml) was stimulated in serum-free medium with coated anti-CD3 (1 μg / ml) and soluble anti-CD28 (1 μg / ml) antibodies, in the presence or absence of 20 μg / ml of an anti-hGARP monoclonal antibody. Thirteen anti-hGARP monoclonals were tested in this assay: the above mentioned nine new monoclonals (MHGARP1 to 9), and commercially available antibody clones Plato-1 (Enzo Life Sciences, catalog No. ALX-804-867), 272G6 (Synaptic Systems, catalog No. 221 111), 50G10 (Synaptic Systems, catalog No. 221 011) and 7B11 (BioLegend, catalog No. 352501). Cells were collected after 24 hours, lysed and submitted to SDS-PAGE under reducing conditions. Gels were blotted on nitrocellulose membranes with the iBlot system (Life Technologies). After blocking, membranes were hybridized with primary antibodies directed against phosphorylated SMAD2 (pSMAD2, Cell Signaling...
example 3
MHGARP8, but not Other Anti-hGARP mAbs, Recognizes a Conformational Epitope that Requires the Presence of TGF-β
Mapping the Regions Recognized by Anti-hGARP Monoclonals
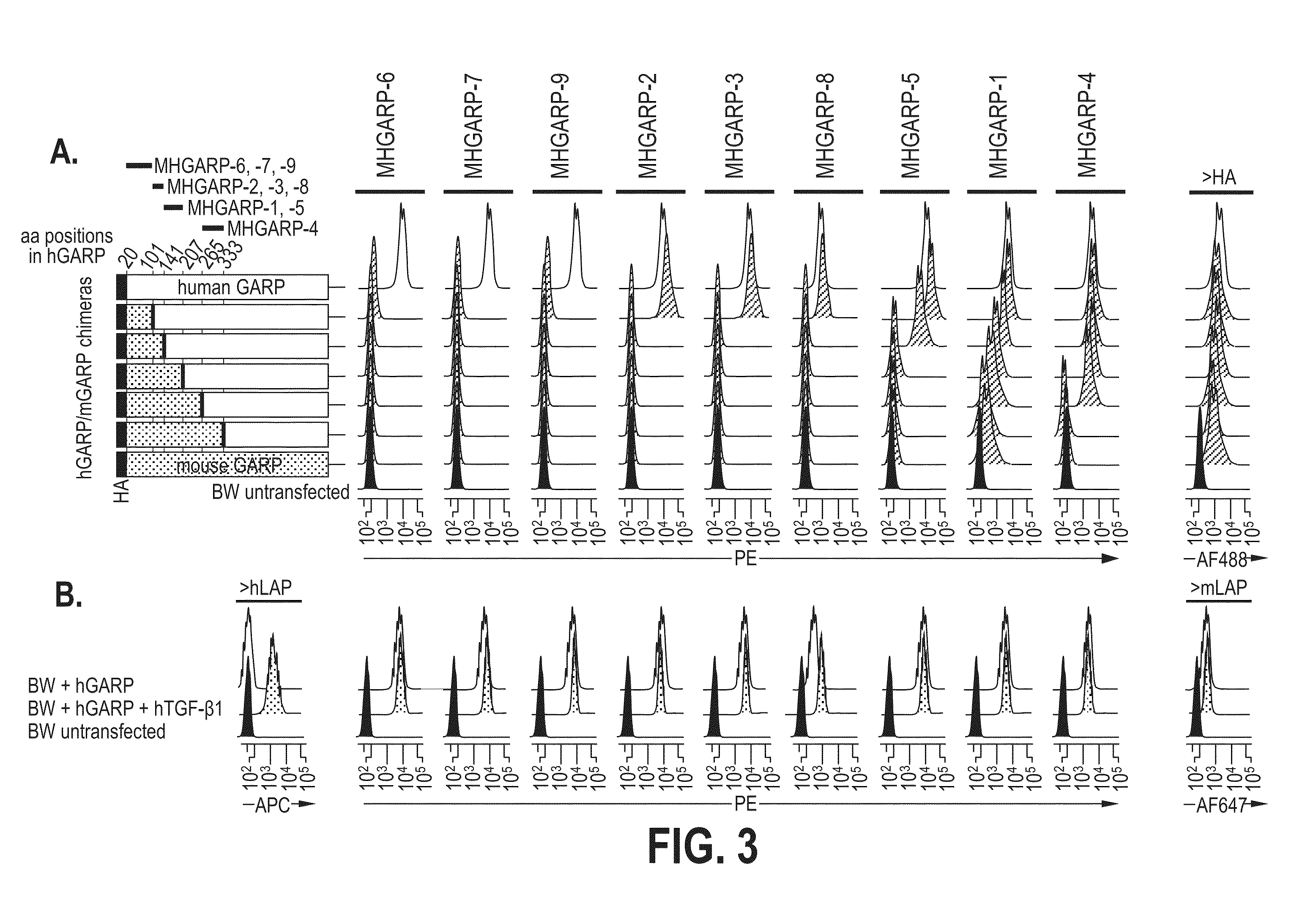
[0329]Murine BW5147 T cells were electroporated with plasmids encoding the HA-tagged proteins schematized in FIG. 3, part A, corresponding to hGARP, mGARP or mGARP / hGARP chimeras. Stable clones selected in neomycin were stained with biotinylated anti-hGARP antibodies (anti-hGARP1 to 9) and streptavidin-PE, with the commercial anti-hGARP antibody (clone Plato-1) and a secondary anti-mIgG2b-AF488, or with an anti-HA antibody and secondary anti-mouse IgG1-AF488. Histograms are gated on live cells. Black histograms show signals on untransfected BW cells, white histograms show signals on BW cells expressing the HA-tagged hGARP, and grey histograms show signals on BW cells expressing HA-tagged mGARP or mGARP / hGARP chimeras.
[0330]Parental BW5147 T cells (BW non-transfected) or clones stably transfected with hGARP alone (BW+hG...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com