Implantable device for the locationally accurate delivery and administration of substances into the pericardium or onto the surface of the heart
a technology of implantable devices and pericardium, which is applied in the field of implantable devices for the location accuracy of the delivery and administration of substances into the pericardium or onto the surface of the heart, and can solve the problems of reduced pumping performance of the heart, decreased thickness of the heart wall, and decreased contractility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048]Embodiments of the invention comprise several components which are explained in more detail in the following sections, it being possible for embodiments of the individual components to be combined with one another.
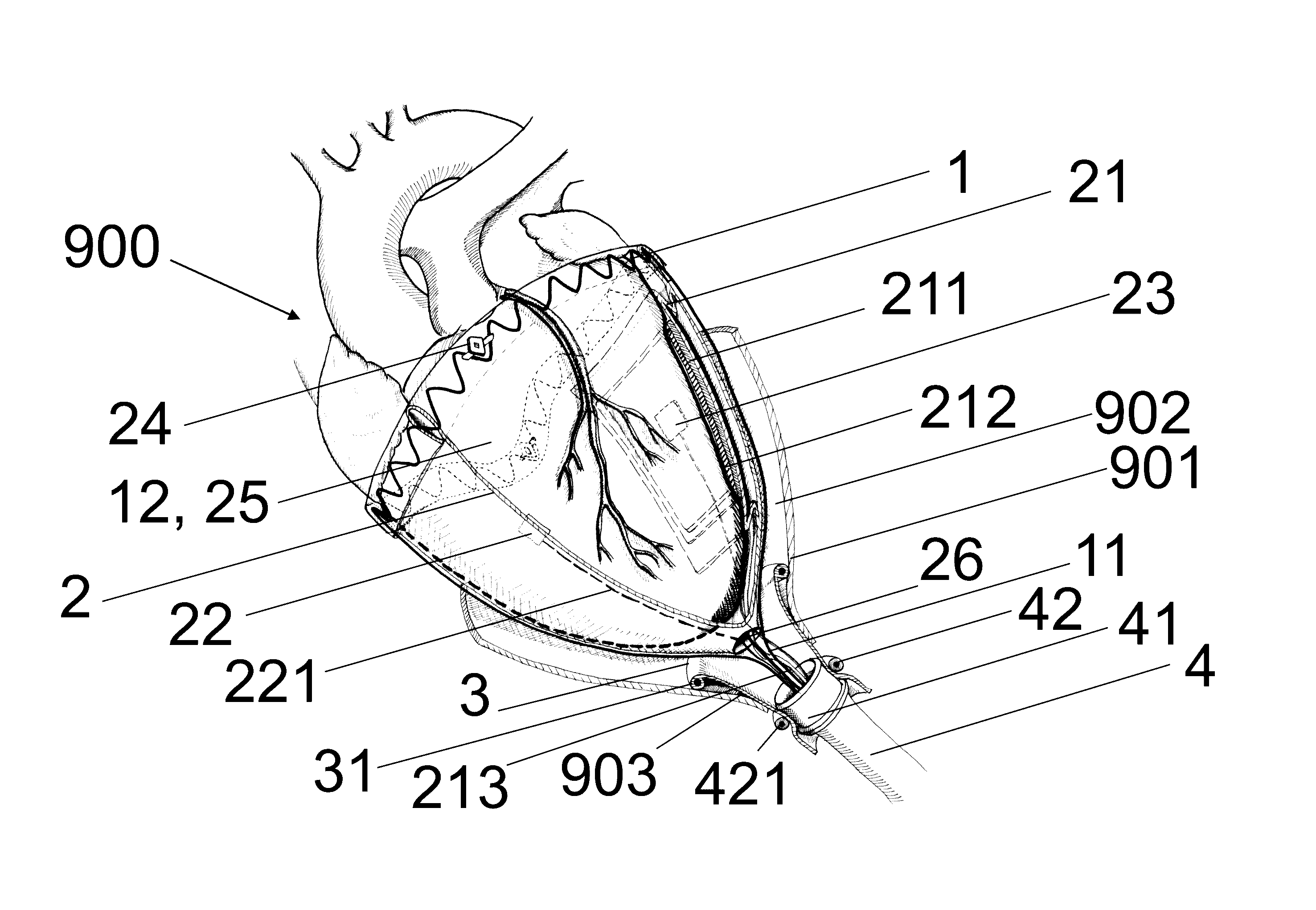
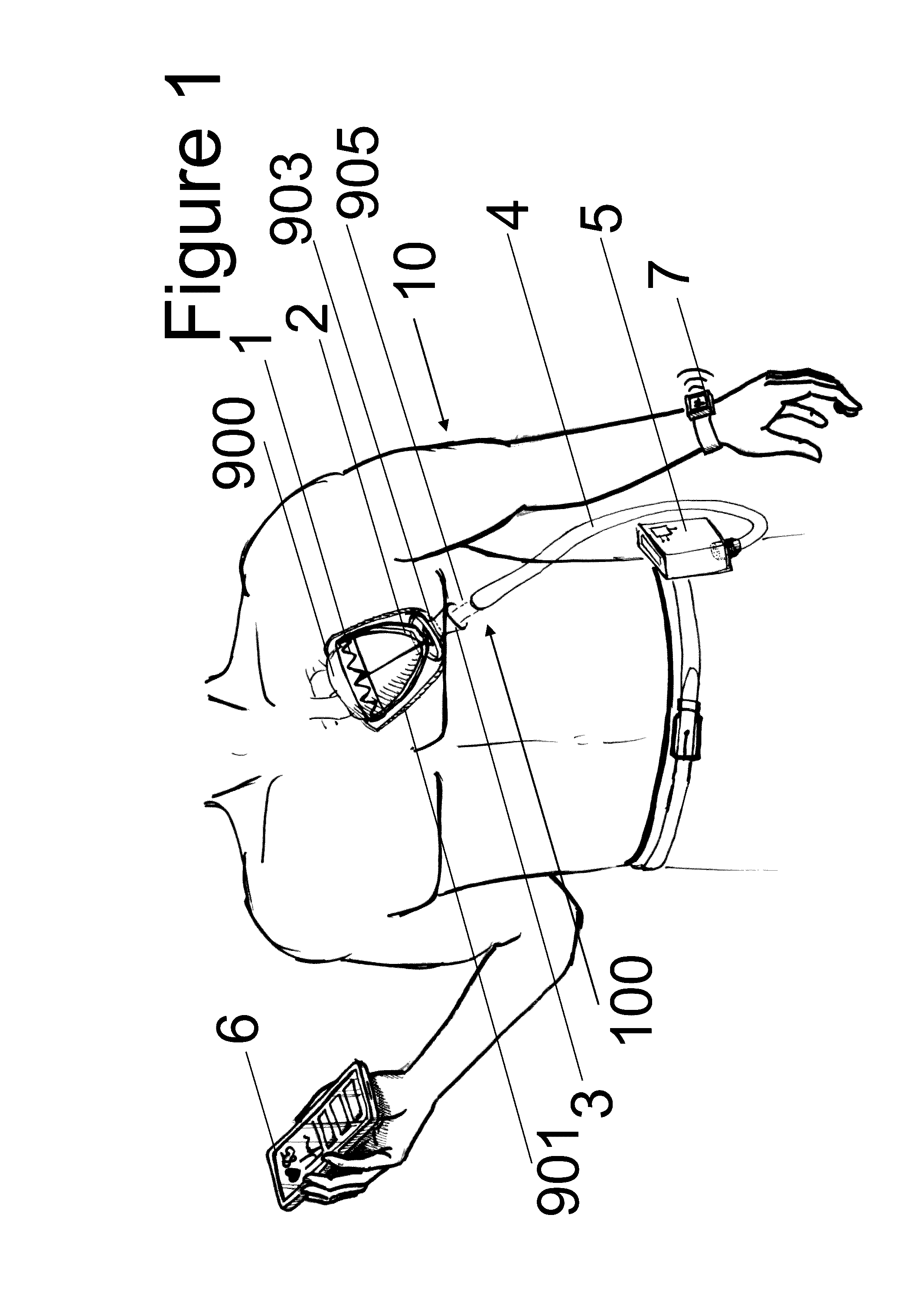
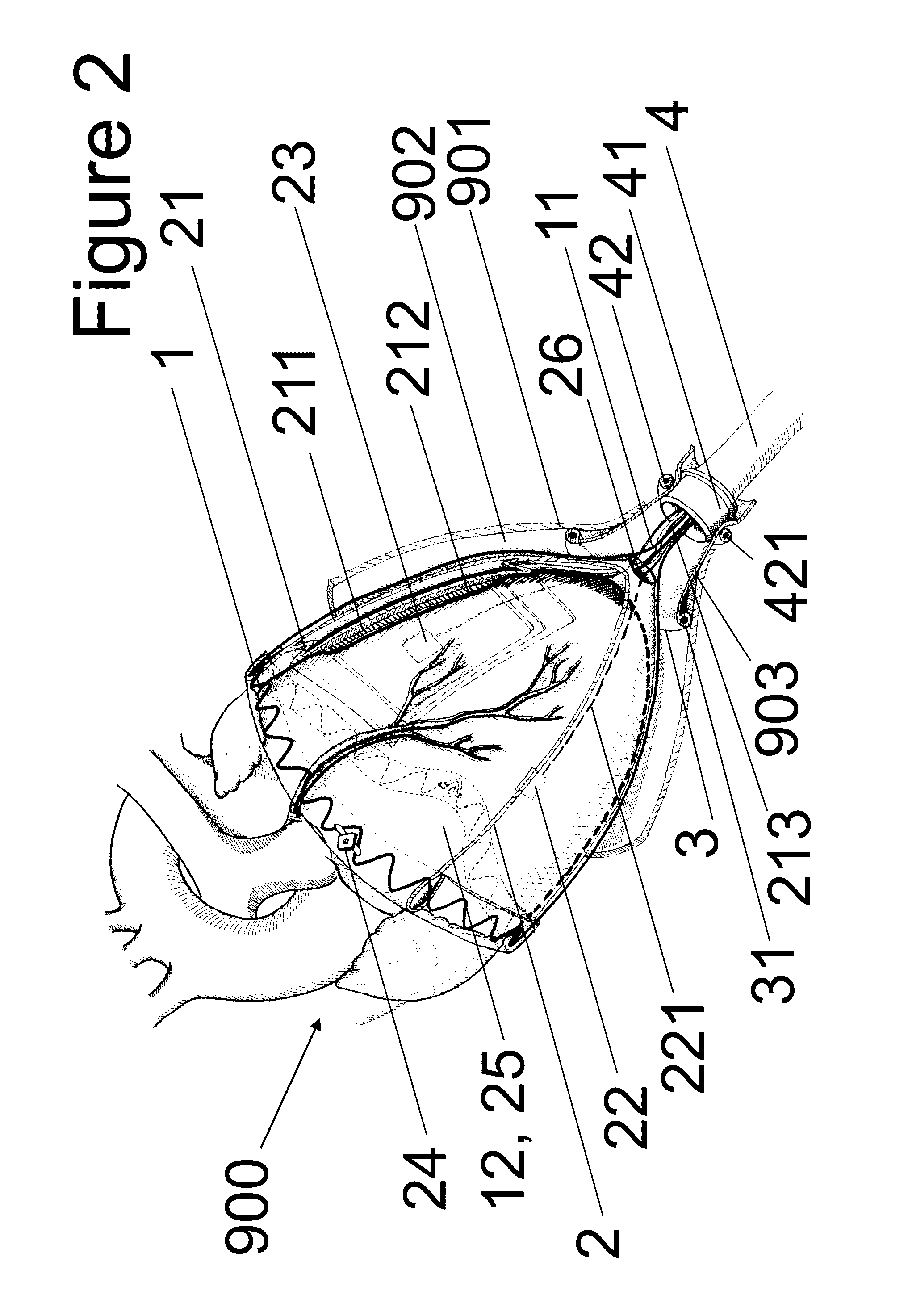
[0049]FIG. 1 shows a human torso with an embodiment (1) of the device according to the invention in the implanted state. The implant (100) comprises a frame structure (1), a sleeve (2) inserted therein, at least one expandable unit (positioned on the sleeve (2) and / or the frame structure (1)) and at least one substance carrier, at least one sensor and at least one electrode. Also ascribed to the implant (100) are a pericardium sluice (3), the implanted part of a cable harness (4) and pneumatic / hydraulic substance lines and electric lines inside the body. The embodiment (1) of the device according to the invention also comprises a supply unit (5). A control unit (6) and a monitoring unit (7) are also depicted. Embodiments of the device according to the invention may b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap