Compositions and methods for treating heart failure
a technology for heart failure and compositions, applied in the field of compositions and methods for treating heart failure, can solve the problems of cardiovascular event patient death risk of patients, etc., and achieve the effects of improving cardiac function, preventing or reducing the severity of cardiac hypertrophy, and increasing the survival time of heart failure patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
c Fusion Proteins
[0342]ActRIIA fusion proteins having the extracellular domain of human ActRIIA fused to a human or mouse Fc domain with a linker in between were generated. The constructs are referred to as ActRIIA-hFc and ActRIIA-mFc, respectively.
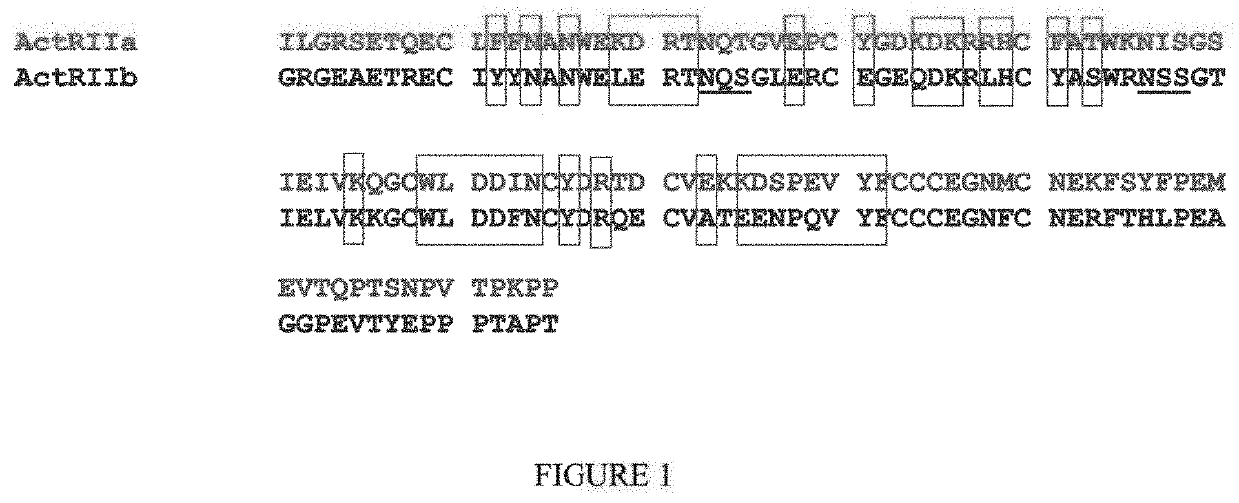
[0343]ActRIIA-hFc is shown below as purified from CHO cell lines (SEQ ID NO: 50):
ILGRSETQECLFFNANWEKDRTNQTGVEPCYGDKDKRRHCFATWKNISGSIEIVKQGCWLDDINCYDRTDCVEKKDSPEVYFCCCEGNMCNEKFSYFPEMEVTQPTSNPVTPKPPTGGGTHTCPPCPAPELLGGPSVFLFPPKPKDTLMI
[0344]The ActRIIA-hFc and ActRIIA-mFc proteins were expressed in CHO cell lines. Three different leader sequences were considered:
(i) Honey bee mellitin (HBML):(SEQ ID NO: 51)MKFLVNVALVFMVVYISYIYA(ii) Tissue plasminogen activator (TPA):(SEQ ID NO: 52)MDAMKRGLCCVLLLCGAVFVSP(iii) Native:(SEQ ID NO: 53)MGAAAKLAFAVFLISCSSGA.
[0345]The selected form employs the TPA leader and has the following unprocessed amino acid sequence:
(SEQ ID NO: 54)MDAMKRGLCCVLLLCGAVFVSPGAAILGRSETQECLFFNANWEKDRTNQTGVEPCYGDKDKRRHCFATWKNISGSIEIV...
example 2
n of ActRIIB-Fc Fusion Proteins
[0350]ActRIIB fusion proteins having the extracellular domain of human ActRIIB fused to a human or mouse Fc domain with a linker in between were constructed. The constructs are referred to as ActRIIB-hFc and ActRIIB-mFc, respectively.
[0351]ActRIIB-hFc is shown below as purified from CHO cell lines (SEQ ID NO: 58):
GRGEAETRECIYYNANWELERTNQSGLERCEGEQDKRLHCYASWRNSSGTIELVKKGCWLDDFNCYDRQECVATEENPQVYFCCCEGNFCNERFTHLPEAGGPEVTYEPPPTAPTGGGTHTCPPCPAPELLGGPSVFLFPPKPKDTLMIS
[0352]The ActRIIB-hFc and ActRIIB-mFc proteins were expressed in CHO cell lines. Three different leader sequences were considered: (i) Honey bee mellitin (HBML), ii) Tissue plasminogen activator (TPA), and (iii) Native: MGAAAKLAFAVFLISCSSGA (SEQ ID NO: 59).
[0353]The selected form employs the TPA leader and has the following unprocessed amino acid sequence (SEQ ID NO: 60):
MDAMKRGLCCVLLLCGAVFVSPGASGRGEAETRECIYYNANWELERTNQSGLERCEGEQDKRLHCYASWRNSSGTIELVKKGCWLDDFNCYDRQECVATEENPQVYFCCCEGNFCNERFTHLPEAGG...
example 3
n of a BMPRII-Fc Fusion Protein
[0372]A homodimeric BMPRII-Fc fusion protein comprising the extracellular domain of human BMPRII fused to a human immunoglobulin G1 Fc domain with a linker was generated. Leader sequences for use with BMPRII-Fc fusion polypeptide include the native human BMPRII precursor leader, MTSSLQRPWRVPWLPWTILLVSTAAA (SEQ ID NO: 68), and the tissue plasminogen activator (TPA) leader.
[0373]The human BMPRII-Fc polypeptide sequence (SEQ ID NO: 69) with a TPA leader is shown below:
(SEQ ID NO: 69)1MDAMKRGLCC VLLLCGAVFV SPGASQNQER LCAFKDPYQQ DLGIGESRIS51HENGTILCSK GSTCYGLWEK SKGDINLVKQ GCWSHIGDPQ ECHYEECVVT101TTPPSIQNGT YRFCCCSTDL CNVNFTENFP PPDTTPLSPP HSFNRDETGG151GTHTCPPCPA PELLGGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP201EVKFNWYVDG VEVHNAKTKP REEQYNSTYR VVSVLTVLHQ DWLNGKEYKC251KVSNKALPAP IEKTISKAKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG301FYPSDIAVEW ESNGQPENNY KTTPPVLDSD GSFFLYSKLT VDKSRWQQGN351VFSCSVMHEA LHNHYTQKSL SLSPGK
[0374]The leader sequence and linker are underlined. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com