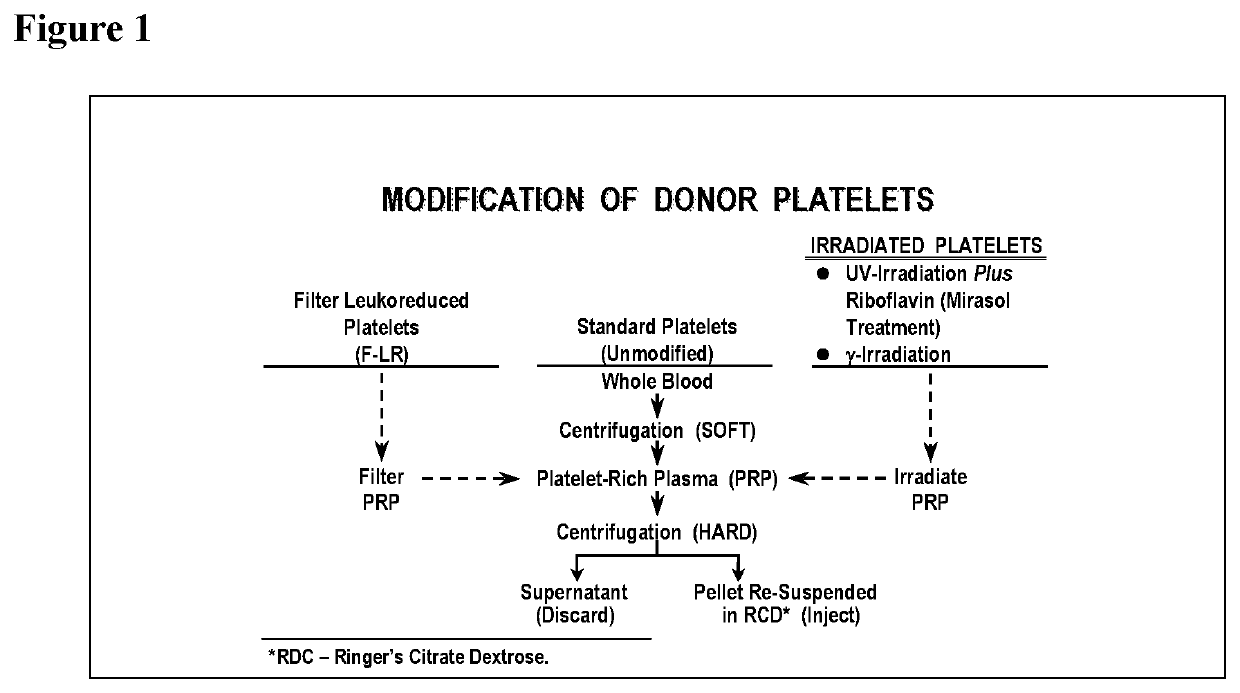
Methods of preventing platelet alloimmunization and alloimmune platelet refractoriness and induction of tolerance in transfused recipients
a technology of alloimmune platelet and transfusion recipient, which is applied in the direction of disinfection, other blood circulation devices, medical devices, etc., can solve the problems of complicated and difficult matching process, platelet refractoriness, and complications of blood product transfusion, so as to prevent alloimmunization, prevent alloimmunization, and prevent alloimmunization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
tal Design and Methods
[0076]The following experimental methods are used in the Examples that follow.
Experimental Design of the Dog Platelet Transfusion Studies
[0077]1) Perform baseline autologous radiolabeled platelet recovery and survival measurements in recipient dogs to ensure that their data is normal.
[0078]2) Select DLA, DRB mismatched and crossmatch negative random donor / recipient pairs.
[0079]3) Prepare platelets weekly from a single random donor.
[0080]4) Donor dog's platelets are unmodified (standard), filter-leukoreduced, γ-irradiated, UV-irradiated plus riboflavin (Mirasol pathogen reduction technology), or treatments are combined.
[0081]5) Donor dog's platelets, after modification, are radiochromium labeled prior to recipient transfusion.
[0082]6) Serial blood samples are drawn from the recipient to determine recovery and survival of the donor dog's radiolabeled platelets.
[0083]7) Recipient receives up to 8 weekly transfusions from their donor or until they become platelet r...
example 2
ness of Different Leukoreduction Methods
[0091]This example provides methods of modifying a donor dog's platelets prior to transfusion in a dog platelet transfusion model that would prevent alloimmune platelet refractoriness. In some aspects, methods of preventing platelet alloimmunization in a dog model could be successfully transferred to patients. Specifically, in some aspects, UV-B irradiation that was 45% successful in preventing alloimmunization in the dog was 81% successful in patients in the largest prevention of platelet alloimmunization trial ever conducted in patients (TRAP Trial). (See Slichter S J, Deeg H J, Kennedy M S. Prevention of platelet alloimmunization in dogs with systemic cyclosporine and by UV-irradiation or cyclosporine-loading of donor platelets. Blood 1987; 69(2):414-418; The Trial To Reduce Alloimmunization To Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet tra...
example 3
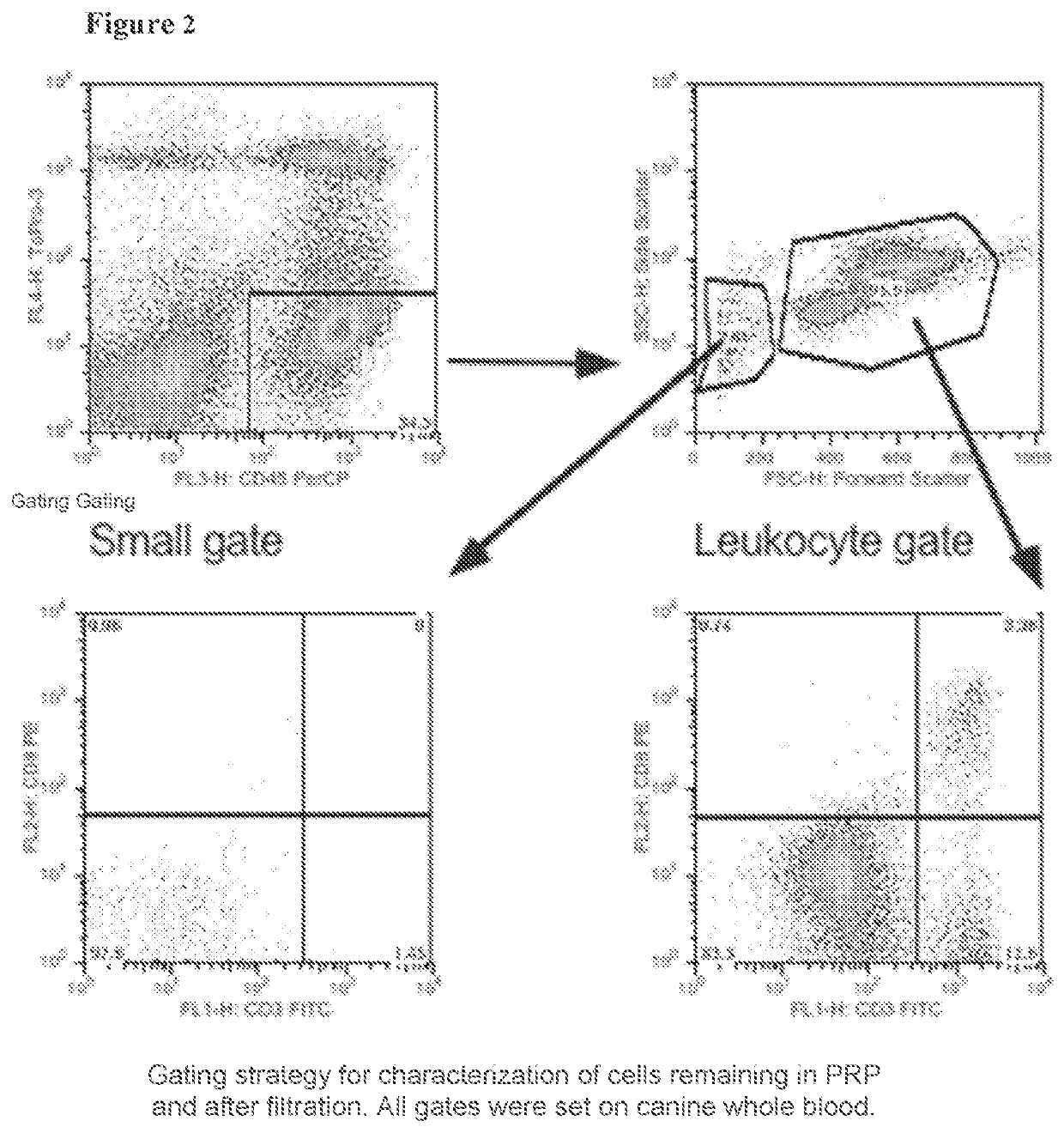
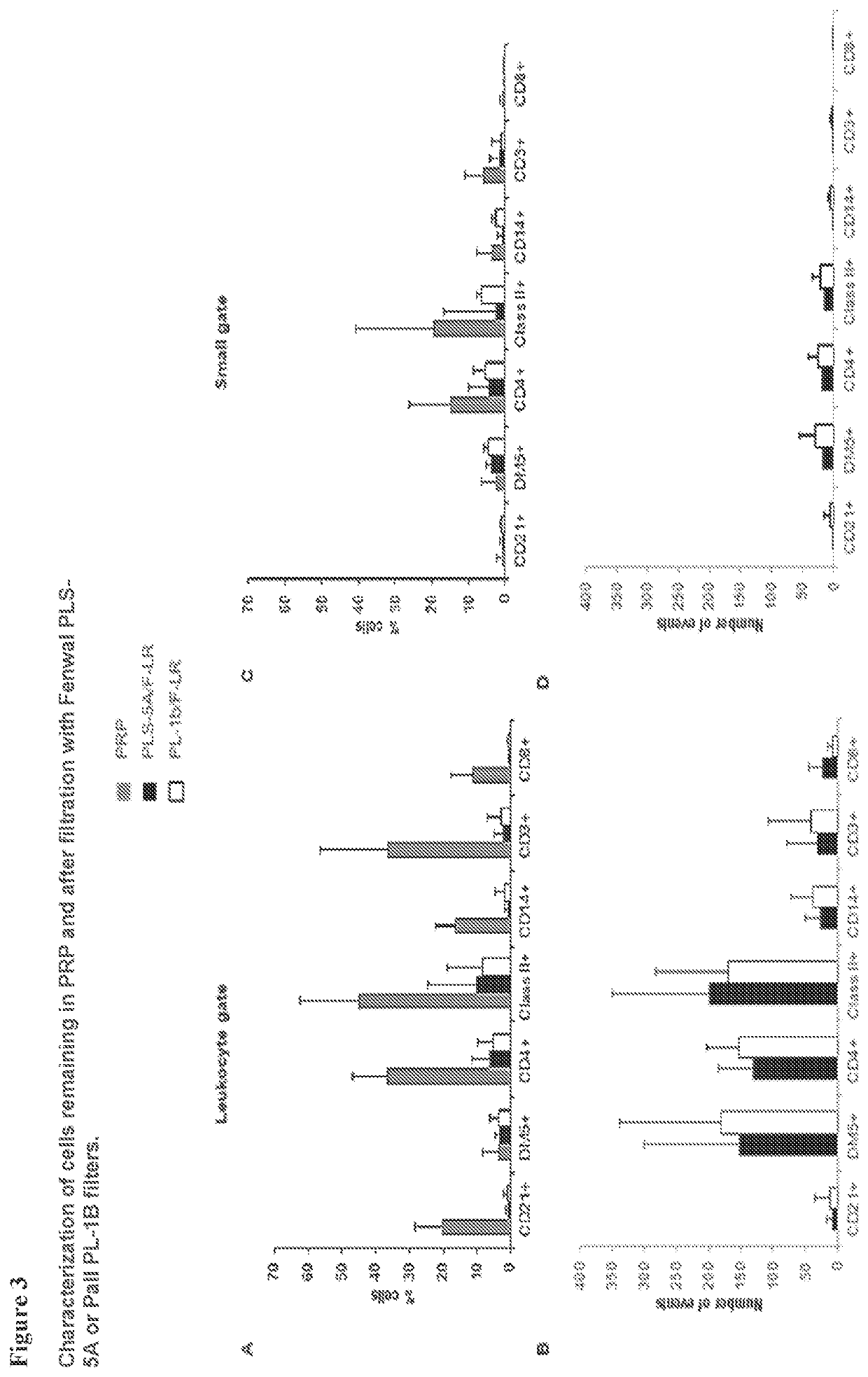
ification
[0098]This Example describes experiments designed to characterize the WBCs that are removed and those that remain after F-LR and combined F-LR / C-LR procedures using monoclonal antibodies specific for canine WBCs.
[0099]The WBC identification method using FACS as described in Example 1 was employed for these studies.
[0100]As expected, the PRP was enriched for lymphocytes for example, B cells (CD21+), T cells (CD3+) and DLA Class II positive (CII+) cells (FIG. 3A). As canine granulocytes, monocytes and T cells express CD4; in the subject analysis CD4 positive cells are a mixture of all these cell types. Both Fenwal PLS-5A and Pall PL-1B filters removed most of the lymphocytes with the percentage of CD8+T cells and B cells in the leukocyte gate below 1% of the total CD45+ cells (FIG. 3A). The analysis of the number of events showed that most of the cells remaining were granulocytes (DM5+ cells) and CII+ cells (FIG. 3B). The percentage of cells and number of events in the low fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com