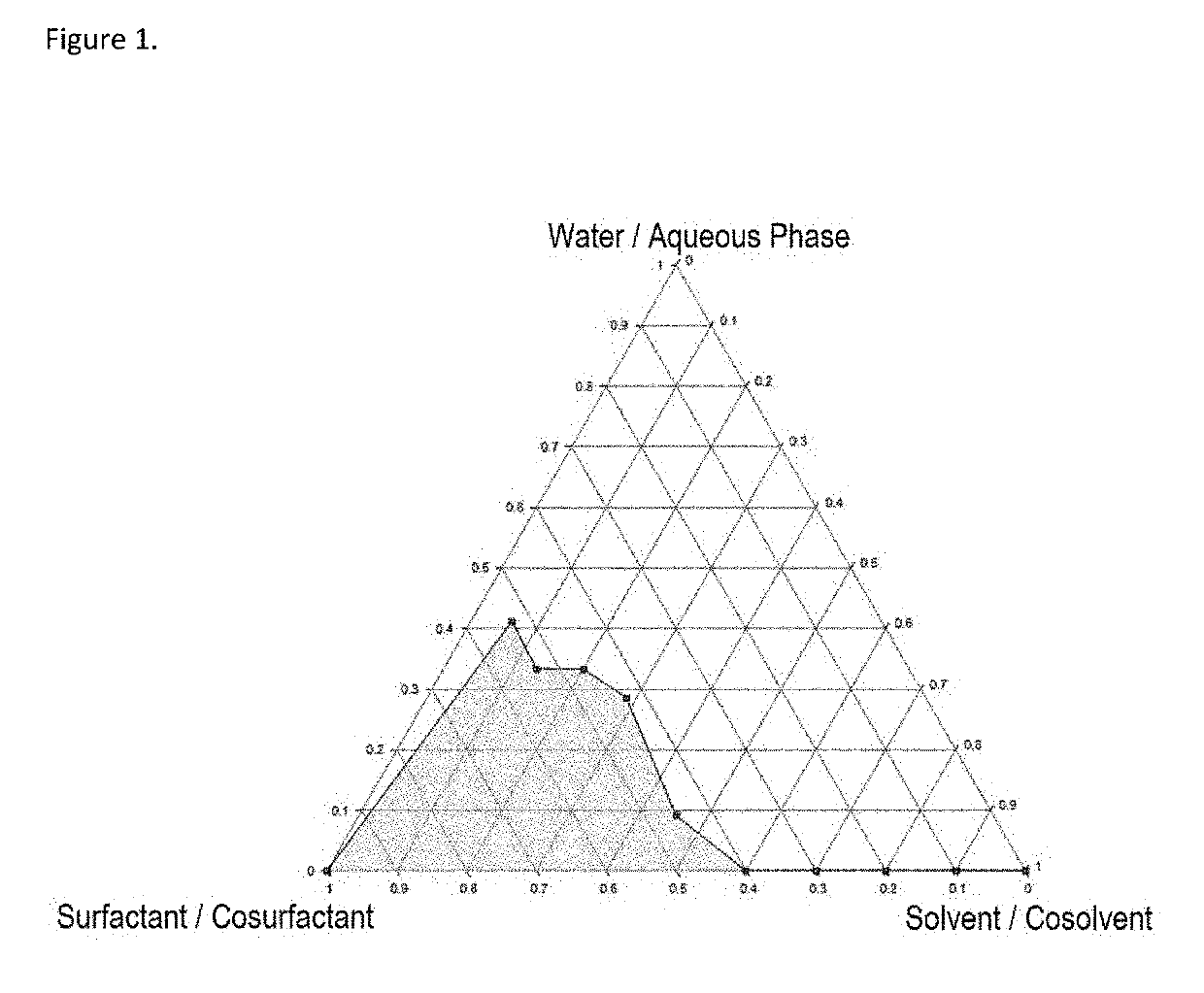
Self-emulsifying formulation of bisphosphonates and associated dosage forms
a technology of self-emulsification and bisphosphonates, which is applied in the field of drug delivery, can solve the problems of very low oral bioavailability of bisphosphonates or bisphosphonic acids, and many patients still complain of heartburn and esophageal burning, so as to improve drug bioavailability and lessen undesirable irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
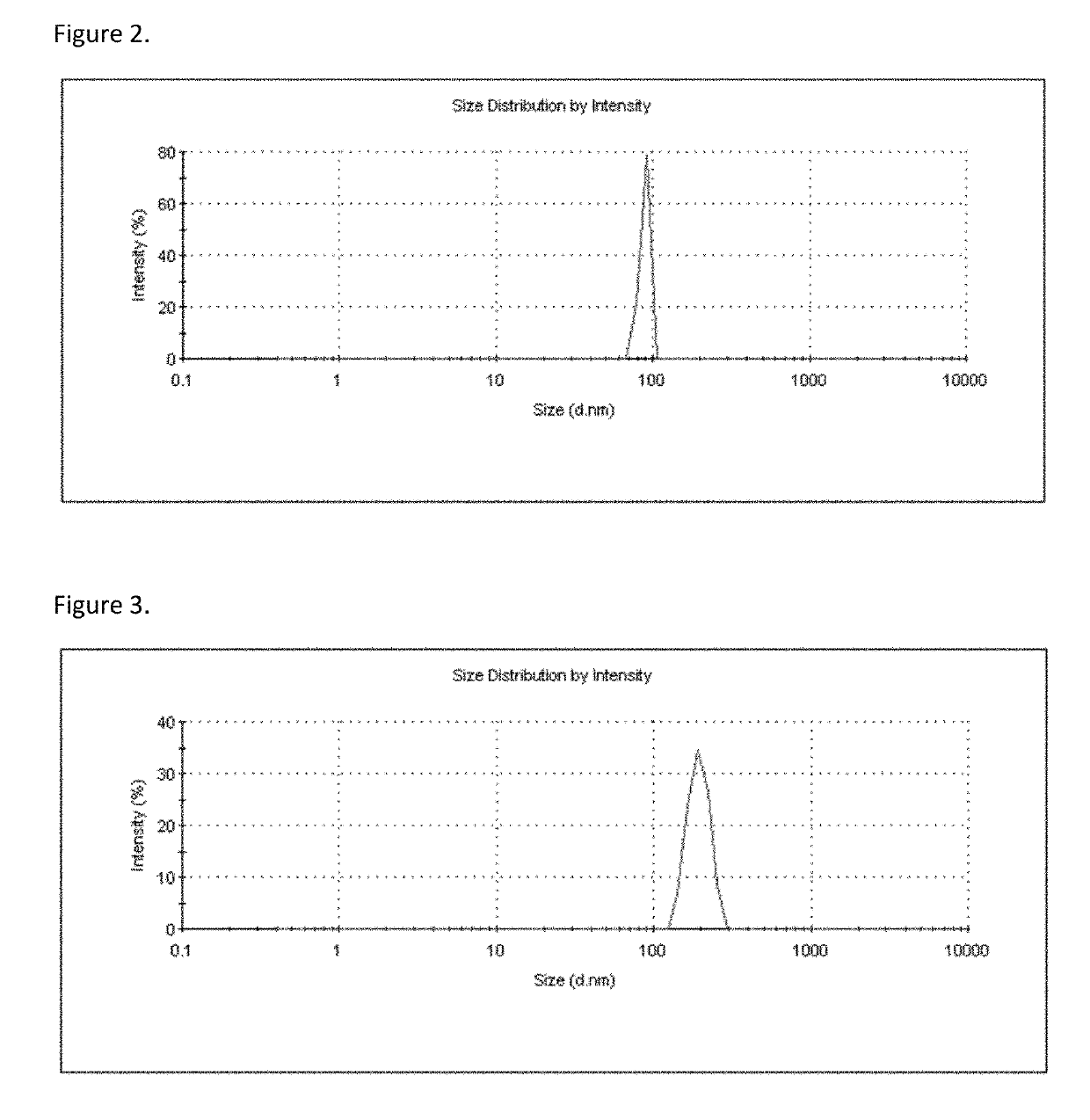
[0103]A self-emulsifying formulation of bisphosphonate was prepared using standard techniques known to those skilled in art. Bisphosphonate was weighed and mixed with solvent, co-solvent, surfactant, and co-surfactant / complexing excipient and thereafter obtained a self-emulsifying drug delivery system. The obtained self-emulsifying preparation was characterized for the particle size by a Malvern dynamic laser diffraction particle size analyzer (FIG. 2).
IngredientQuantityFunctionAlendronate 1 gAPIPEG 40020 gSolventBenzyl alcohol17 gCo-solventSpan 8025 gSurfactantLecithin 2 gCo-surfactant / ComplexingExcipientPurified water100 g Co-solvent
example 2
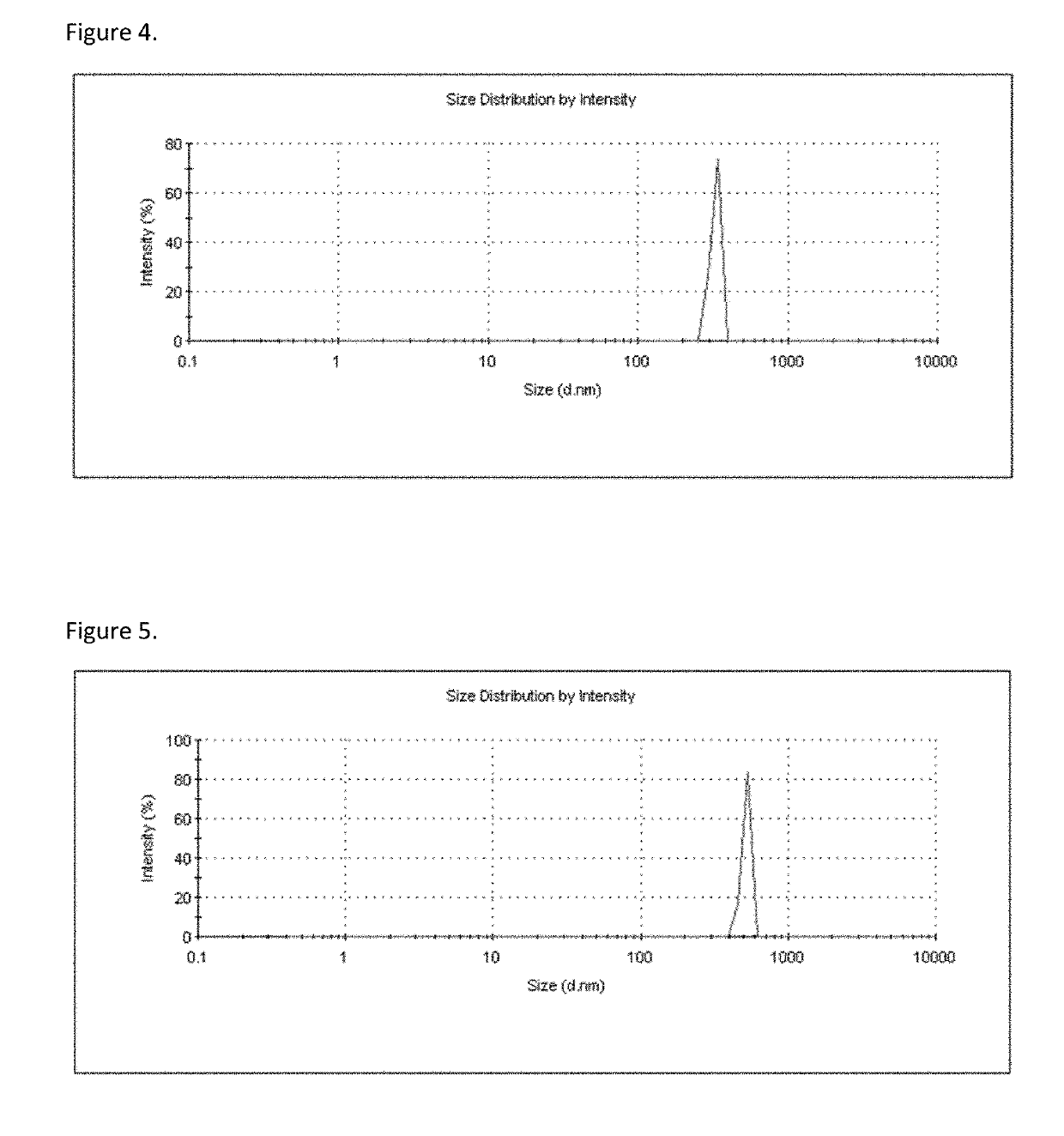
[0104]A self-emulsifying formulation of bisphosphonate was prepared using standard techniques known to those skilled in art. Bisphosphonate was weighed and mixed with solvent, co-solvent, surfactant, and co-surfactant / complexing excipient and thereafter obtained a self-emulsifying drug delivery system. The obtained self-emulsifying preparation was characterized for the particle size by a Malvern dynamic laser diffraction particle size analyzer (FIG. 3).
IngredientQuantityFunctionAlendronate 1 gAPIMiglyol 812 95 gSolventTween 80100 gSurfactantLecithin 2 gCo-surfactant / ComplexingExcipientPurified water100 gCo-solvent
example 3
[0105]A self-emulsifying formulation of bisphosphonate was prepared using standard techniques known to those skilled in art. Bisphosphonate was weighed and mixed with solvent, co-solvent, surfactant, and co-surfactant / complexing excipient and thereafter obtained a self-emulsifying drug delivery system. The obtained self-emulsifying preparation was characterized for the particle size by a Malvern dynamic laser diffraction particle size analyzer (FIG. 4).
IngredientQuantityFunctionAlendronate 1 gAPIPEG 40020 gSolventBenzyl alcohol17 gCo-solventMiglyol 829170 g Co-solventSpan8025 gSurfactantLecithin 2 gCo-surfactant / ComplexingExcipientPurified water100 g Co-solvent
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| droplet size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com