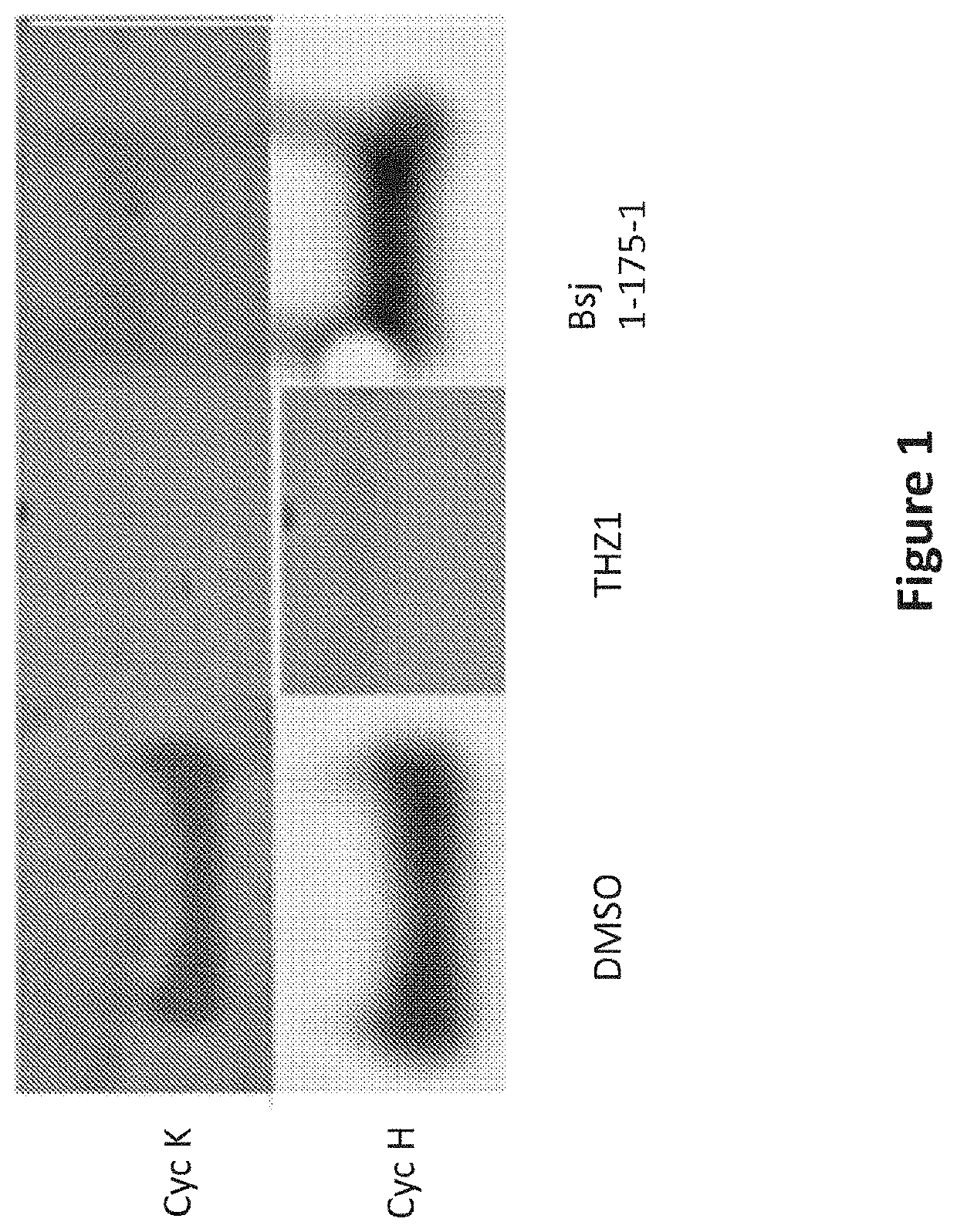
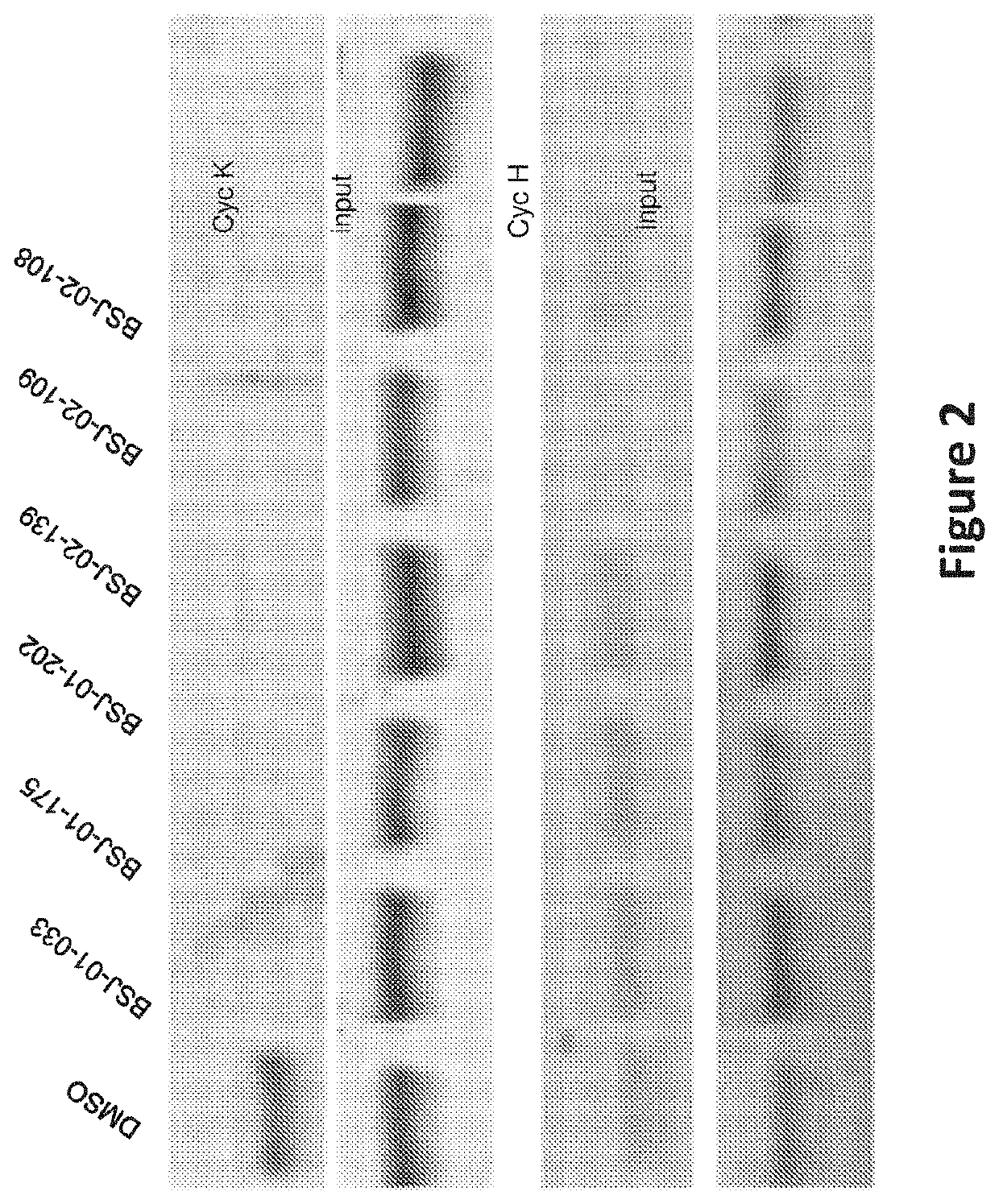
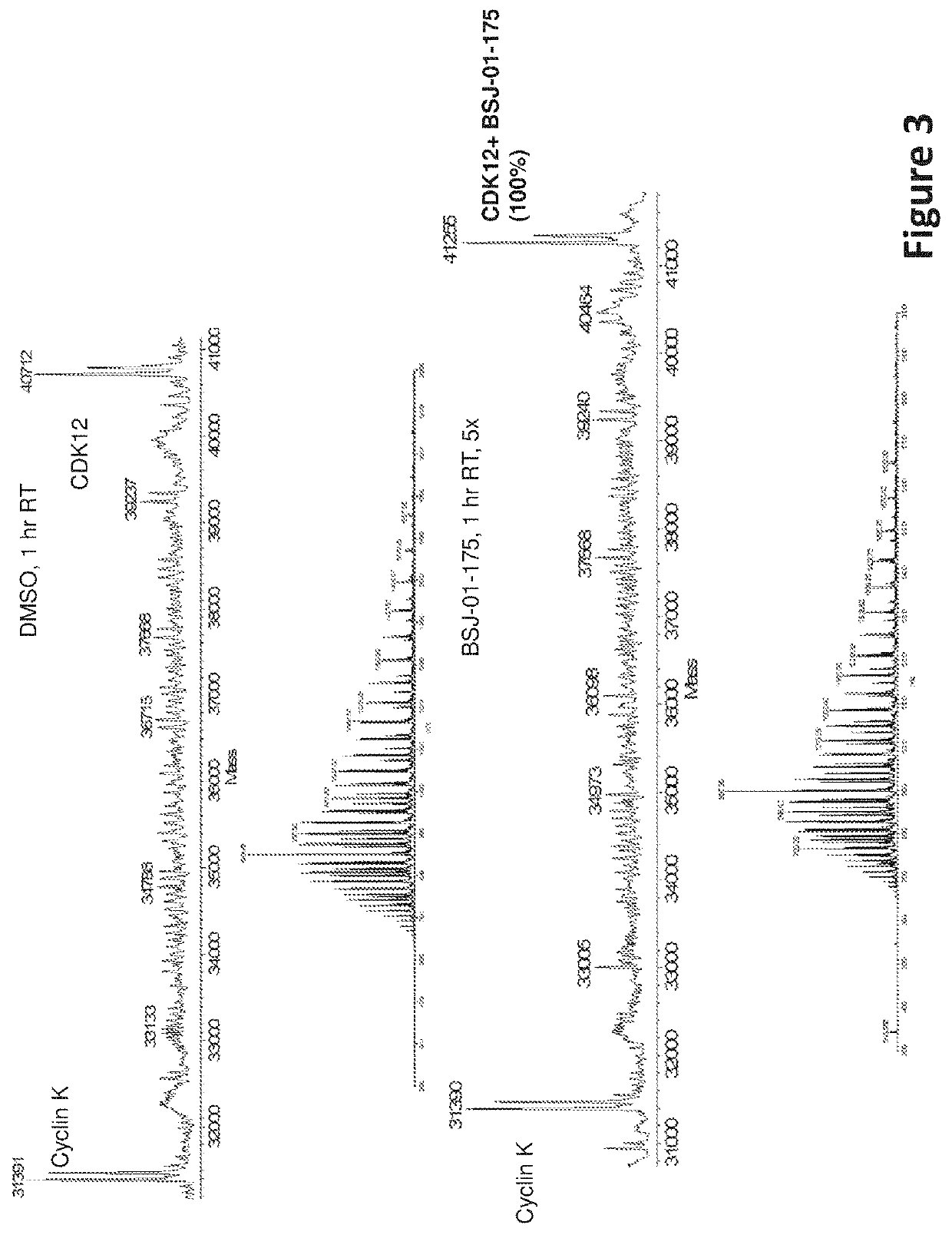
Inhibitors of cyclin-dependent kinase 12 (CDK12) and uses thereof
a cyclin-dependent kinase and inhibitor technology, applied in the field of inhibitors of cyclin-dependent kinase 12 (cdk12), can solve the problems of prolonged disruption of transcription and induction of apoptosis of a diverse subset of cancer cell lines, unclear mechanism, and inability to precisely determine the site of cdk12 phosphorylation on ctd, etc., to achieve the effect of reducing or avoiding symptoms or
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of N-(4-((1R,3R)-3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)cyclohexyloxy)phenyl)acrylamide (BSJ-01-033)
[0385]
(1R,3R)-3-(4-nitrophenoxy)cyclohexanamine
[0386]
[0387]To a suspension of NaH (0.8 g, 8.25 mmol) in 3.0 mL of anhydrous DMF was added (1R,3R)-3-aminocyclohexanol HCl salt (0.5 g, 3.3 mmol) slowly at 0° C. and kept stirring for 0.5 h, then 1-fluoro-4-nitrobenzene (0.465 g, 3.3 mmol) was added. The reaction mixture was kept stirring at 0° C. for another 0.5 h, then warm to room temperature and kept stirring for 2 h. 1.0 mL of H2O was added dropwise to quench the reaction. The mixture was extracted with DCM (100 mL), washed with brine (3×50 mL), dried (anhydrous Na2SO4), filtered and concentrated under reduced pressure. The residue was purified by chromatography on silica gel to give the title compound (623 mg, 80%) as a brown solid. LC-MS (m / z): 237 [M+H]+.
5-chloro-N-((1R,3R)-3-(4-nitrophenoxy)cyclohexyl)-4-(1-(phenylsulfonyl)-1H-indol-3-yl)pyrimidin-2-amine
[0388]
[0389]3-(...
example 2
of (E)-N-(4-((1R,3R)-3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)cyclohexyloxy)phenyl)-4-(dimethylamino)but-2-enamide (BSJ-01-175)
[0396]
[0397]To a cold solution (0° C.) of N-((1R,3R)-3-(4-aminophenoxy)cyclohexyl)-5-chloro-4-(1H-indol-3-yl)pyrimidin-2-amine (15 mg, 0.035 mmol) and 0.1 mL of DIPEA in 2.0 mL of anhydrous CH3CN was added a solution of (E)-4-bromobut-2-enoyl chloride (6.34 mg, 0.035 mmol) in 1.0 mL of DCM dropwise. After 0.5 h at 0° C., a 2M solution of dimethylamine in THF (1.0 mL) was added and the mixture was stirred for 1 h at 0° C. Then 1.5 mL of DMSO was added, followed by removal of the low boiling point solvents under reduced pressure. The residue was purified by prep-HPLC (MeOH / H2O, 0.05% TFA) to give the title compound (11 mg, 58%) as a light yellow solid after lyophilisation. LC-MS (m / z): 545 [M+H]+. 1H NMR (500 MHz, DMSO-d6) δ 10.92 (dd, J=10.4, 5.6 Hz, 1H), 10.38 (s, 1H), 8.65 (d, J=8.4 Hz, 2H), 8.36 (s, 1H), 7.59 (d, J=8.7 Hz, 2H), 7.54 (d, J=8.1 Hz, 1...
example 3
of (E)-N-(4-(3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)cyclohexyloxy)phenyl)-4-(dimethylamino)but-2-enamide (BSJ-01-193)
[0398]
3-(4-nitrophenoxy)cyclohexanamine
[0399]
[0400]To a suspension of NaH (0.8 g, 8.25 mmol) in 3.0 mL of anhydrous DMF was added 3-aminocyclohexanol HCl salt (0.5 g, 3.3 mmol) slowly at 0° C. and kept stirring for 0.5 h, then 1-fluoro-4-nitrobenzene (0.465 g, 3.3 mmol) was added. The reaction mixture was kept stirring at 0° C. for another 0.5 h, then warm to room temperature and kept stirring for 2 h. 1.0 mL of H2O was added dropwise to quench the reaction. The mixture was extracted with DCM (100 mL), washed with brine (3×50 mL), dried (anhydrous Na2SO4), filtered and concentrated under reduced pressure. The residue was purified by chromatography on silica gel to give the title compound (623 mg, 80%) as a brown solid. LC-MS (m / z): 237 [M+H]+.
5-chloro-N-(3-(4-nitrophenoxy)cyclohexyl)-4-(1-(phenylsulfonyl)-1H-indol-3-yl)pyrimidin-2-amine
[0401]
[0402]3-(2,5-dic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com