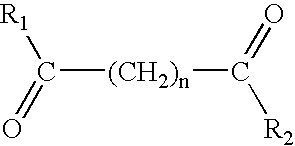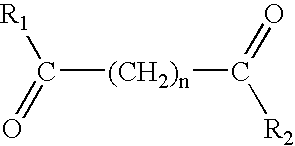Potent inducers of terminal differentiation and methods of use thereof
a terminal differentiation and potent technology, applied in the field of terminal differentiation potent inducers, can solve the problems of limited potent efficacy of hmba, and the inability of symmetrical dimers such as hmba and related compounds to be the best cytodifferentiating agents, and achieve the effect of inhibiting their proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
The present invention provides the compound having the structure: ##STR15##
wherein each of R.sub.1 and R.sub.2 are independently the same as or different from each other; when R.sub.1 and R.sub.2 are the same, each is a substituted or unsubstituted arylamino, cycloalkylamino, pyridineamino, piperidino, 9-purine-6-amine, or thiozoleamino group; when R.sub.1 and R.sub.2 are different, R.sub.1 =R.sub.3 --N--R.sub.4, wherein each of R.sub.3 and R.sub.4 are independently the same as or different from each other and are a hydrogen atom, a hydroxyl group, a substituted or unsubstituted, branched or unbranched alkyl, alkenyl, cycloalkyl, aryl, alkyloxy, aryloxy, arylalkyloxy, or pyridino group, or R.sub.3 and R.sub.4 bond together to form a piperidine group and R.sub.2 is a hydroxylamino, hydroxyl, amino, alkylamino, dialkylamino or alkyloxy group; and n is an integer from about 4 to about 8.
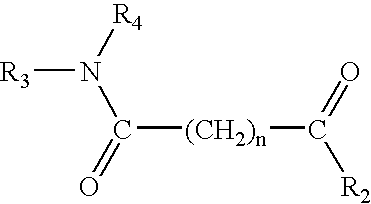
The present invention also provides the compound above having the structure: ##STR16##
wherein each o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com