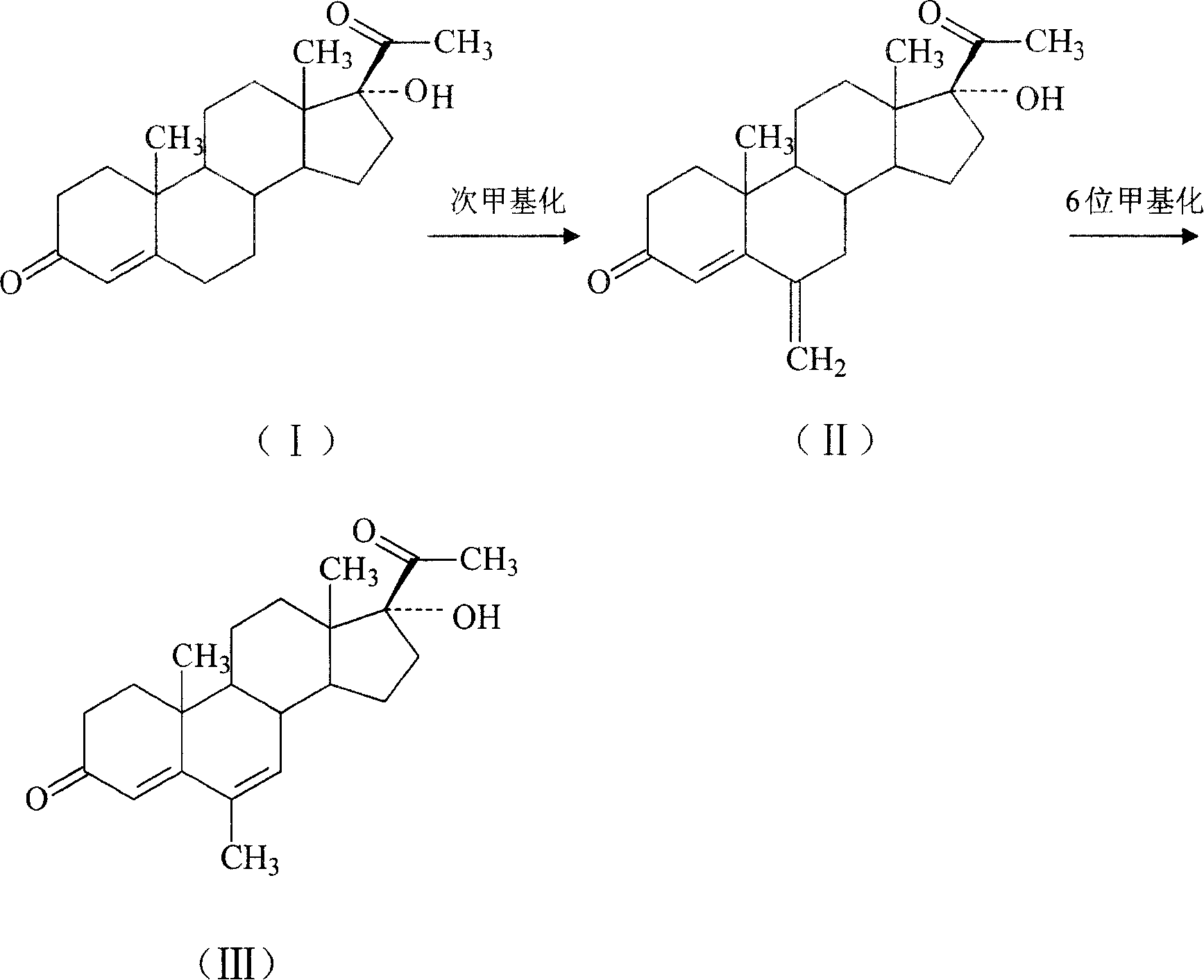
Chemical synthesis of free megestrol
A technology for chemical synthesis and megestrol, applied in the fields of organic chemistry, steroids, etc., can solve the problems of incomplete methylation transposition, poor quality of reaction products, long reaction time, etc., to achieve easy industrialization, reduce Generation of by-products, the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 17α-Hydroxyprogesterone 30KG is subjected to methine reaction to obtain 23KG of methine. 260L of dimethylformamide was pumped into the reaction tank, dropped into 23KG of methine, 12KG of 5% palladium-carbon catalyst, 1200ml of cyclohexene, was incubated at 70 DEG C for 1.5 hours, filtered, and concentrated while filtering. The weight is 1.5 times the weight of the input methine, cooled to below 5°C for centrifugation, rinsed with warm water at 50°C for 1 hour, and dried to obtain crude free megestrol, which was purified to obtain 16.1KG with a yield of 70.0%.
Embodiment 2
[0022] 17α-Hydroxyprogesterone 30KG is subjected to methine reaction to obtain 23KG of methine. 240L of dimethylformamide was pumped into the reaction tank, dropped into 23KG of methine, 12KG of 5% palladium-carbon catalyst, 1200ml of cyclohexene, was incubated at 60 DEG C for 3 hours, filtered, and concentrated while filtering. The weight is 1.5 times the weight of the input methine, cooled to below 5°C for centrifugation, rinsed with 50°C warm water for 0.5 hours, and dried to obtain a crude free megestrol.
Embodiment 3
[0024] 17α-Hydroxyprogesterone 30KG is subjected to methine reaction to obtain 23KG of methine. 260L of dimethylformamide was pumped into the reaction tank, dropped into methine 23KG, 5% palladium-carbon catalyst 12KG, cyclohexene 1100ml, was incubated at 55 DEG C for 2.5 hours, filtered, concentrated while filtering, the mother liquor after the concentration was concentrated. The weight is 1.5 times the weight of the input methine, cooled to below 5°C for centrifugation, rinsed with 50°C warm water for 0.5 hours, and dried to obtain a crude free megestrol, which was purified to obtain 16.4KG with a yield of 71.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com