Liposome vitamin A acid aerosol for treating chronic obstructive pulmonary disease
A chronic obstructive pulmonary, retinoic acid technology, used in aerosol delivery, respiratory diseases, organic active ingredients, etc., can solve the imbalance of lung ventilation/blood flow, sputum aggravating dyspnea, limited clinical use, etc. problem, to achieve the effect of reducing the side effects of retinoic acid, convenient preparation and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
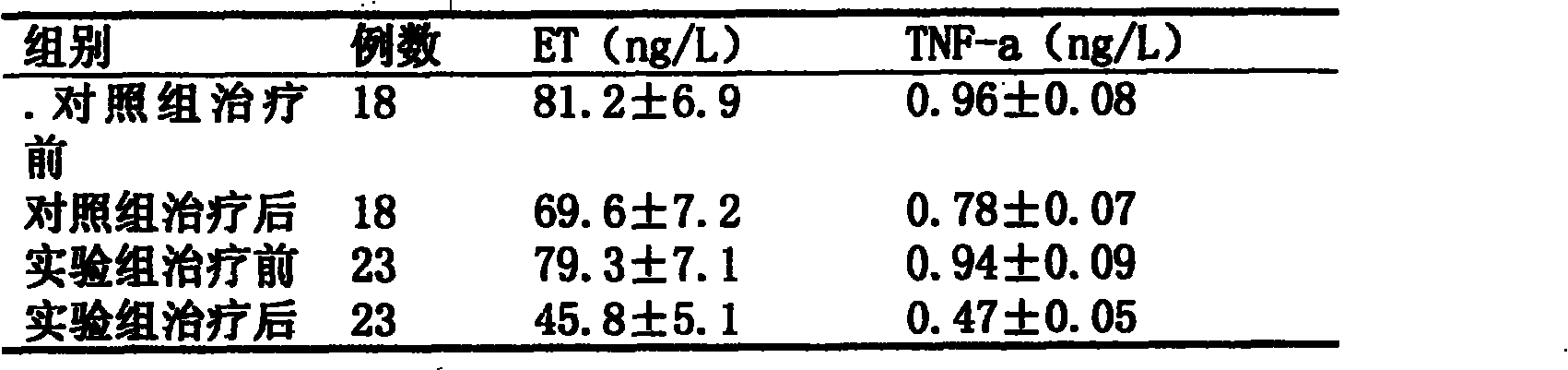
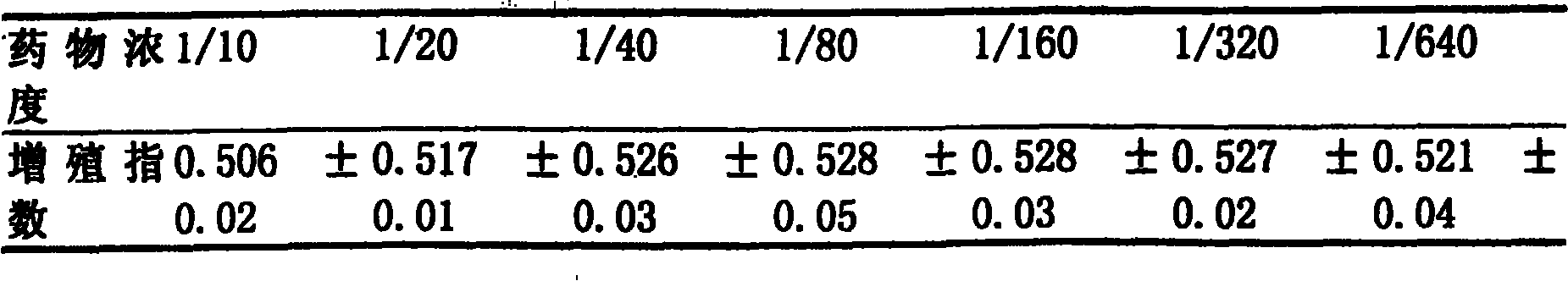
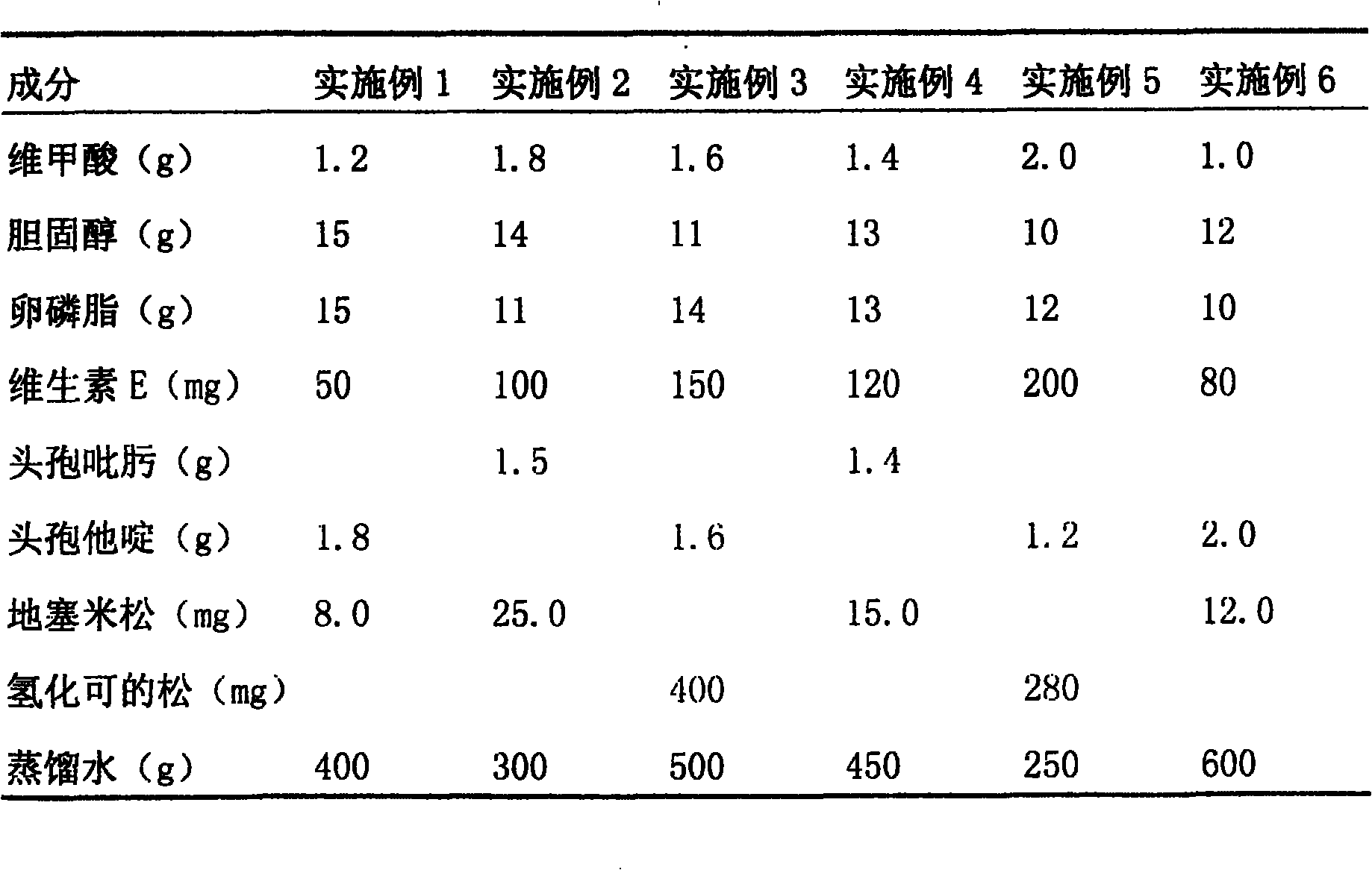
[0029] Retinoic acid is compatible with cefepime and dexamethasone to make an aerosol, which has high local anti-inflammatory activity and glucocorticoid receptor specificity. After inhalation, it has a significant anti-inflammatory effect in the lungs, which can reduce symptoms and prevent inflammation. The condition worsened, and most of the patients in Examples 1-6 had remission within 1 to 2 weeks. But also can add other ingredients, to achieve the purpose of effective treatment.
[0030]
[0031] In the above-mentioned examples, retinoic acid, cholesterol, lecithin, and vitamin E were first dissolved in 200ml of ethanol, slowly injected into the PBS buffer solution with a pH of 7.2 to 7.4 under constant temperature stirring, and further passed through micropores with a pore size of 0.1 μm. filter membrane, after freeze-drying, fill the sample with nitrogen and seal to obtain retinoic acid liposomes. Then retinoic acid liposome is mixed with cefepime or ceftazidime, de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com